2023-03-27 ワシントン州立大学(WSU)
トリコモナス膣炎は、クラミジアや淋病よりも一般的で、約70%の感染者に症状は現れず、症状がなくても、HIVへの感受性の増加、男性の前立腺がん、女性の不妊や妊娠合併症などの健康問題に関連している。
これまでの検査法は、女性の診断に焦点を当てており、結果を得るまでに時間がかかり、専門的な訓練と装置が必要であるが、新しい検査キットは、わずか5分で結果を出すことができ、20ドル以下で製造できる。
この検査キットには、専門的な訓練と装置は必要なく、指の刺し傷から血液を一滴採取し、トリコモナス膣炎特有の抗体を検出する。抗体があれば感染が確認され、治療につながる。この検査キットが世界保健機関の「ASSURED」基準を満たし、低資源国家での使用も目的としている。
<関連情報>
- https://news.wsu.edu/press-release/2023/03/27/finger-prick-test-developed-for-trich-a-common-undiagnosed-sti/
- https://www.mdpi.com/2076-0817/12/1/77
最も一般的で非ウイルス性の性感染症であるトリコモナス膣炎のポイントオブケア診断薬 Point-of-Care Diagnostic for Trichomonas vaginalis, the Most Prevalent, Non-Viral Sexually Transmitted Infection
John F. Alderete and Hermes Chan
Pathogens Published: 3 January 2023
DOI:https://doi.org/10.3390/pathogens12010077
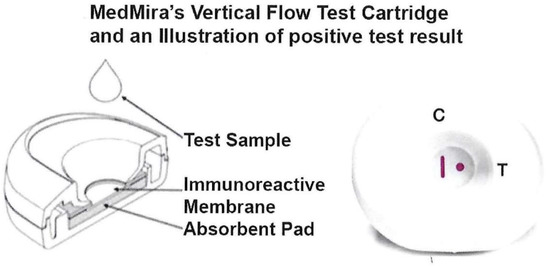
Abstract
A point-of-care (POC) diagnostic is needed for both women and men to establish universal screening and surveillance for the number one, non-viral sexually transmitted infection (STI) caused by Trichomonas vaginalis. We developed a POC diagnostic for this STI using the MedMira Rapid Vertical Flow (RVF®) Technology test cartridge with a membrane that includes a Vertical procedural/reagent control line (referred to as CVL) and spotted with 1 µg of a 72.4-kDa truncated version of α-actinin called ACT::SOE3. This protein is a specific diagnostic target for antibody in sera of individuals with trichomoniasis. Serum antibody to ACT::SOE3 is a positive reaction with the test spot. Specificity of ACT::SOE3 was revealed with monoclonal antibodies (MAbs) generated to ACT::SOE3. Addition of negative control serum with MAb 67B reactive to ACT::SOE3 shows detection of both ACT::SOE3 and the CVL. Only positive sera of individuals had antibody reactive with ACT::SOE3 and detected the presence of the spot and the CVL. Negative control sera were unreactive with ACT::SOE3 and only showed the presence of the CVL. Importantly, to show proof-of-principle for POC application, ACT::SOE3 was detected with the positive patient sera spiked with whole blood. Finally, packaged cartridges stored with desiccant packs at 37 °C for one year gave identical results with the positive and negative human sera. Our results show the validity of this new POC serodiagnostic for this STI.