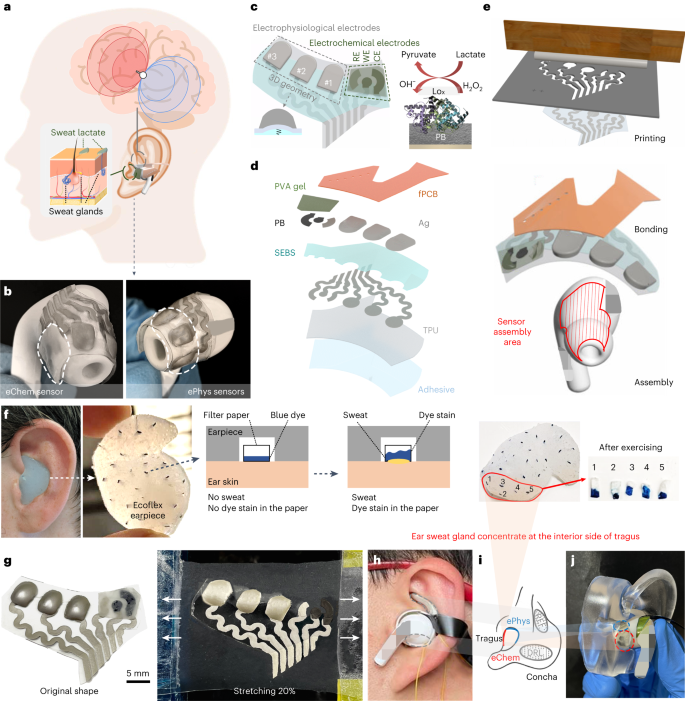
2023-09-25 ミシガン大学
◆この研究により、致命的なけいれん時の呼吸、心拍数、脳活動の相互作用に関する洞察が明らかになり、DEPDC5関連てんかんのSUDEPのリスクが高まるプロセスの理解に道を開きました。
◆モデルは、哺乳動物の生物学的特性に合わせて設計され、SUDEP前の身体内での変化を調べるのに役立ち、SUDEPのリスクを予測するためのバイオマーカーとしても活用できる可能性があります。
<関連情報>
- https://news.umich.edu/u-m-scientists-develop-a-new-model-for-understanding-sudden-death-in-epilepsy/
- https://onlinelibrary.wiley.com/doi/10.1002/ana.26773
DEPDC5関連てんかんモデルマウスにおける予期せぬ突然死と呼吸器障害 Sudden Unexpected Death in Epilepsy and Respiratory Defects in a Mouse Model of DEPDC5-Related Epilepsy
Hsin-Yi Kao, Yilong Yao, Tao Yang, Julie Ziobro, Mary Zylinski, Mohd Yaqub Mir, Shuntong Hu, Runnan Cao, Nurun Nahar Borna, Rajat Banerjee, Jack M. Parent, Shuo Wang, Daniel K. Leventhal, Peng Li, Yu Wang
Annals of Neurology Published: 22 August 2023
DOI:https://doi.org/10.1002/ana.26773

Abstract
Objectives
DEPDC5 is a common causative gene in familial focal epilepsy with or without malformations of cortical development. Its pathogenic variants also confer a significantly higher risk for sudden unexpected death in epilepsy (SUDEP), providing opportunities to investigate the pathophysiology intersecting neurodevelopment, epilepsy, and cardiorespiratory function. There is an urgent need to gain a mechanistic understanding of DEPDC5-related epilepsy and SUDEP, identify biomarkers for patients at high risk, and develop preventive interventions.
Methods
Depdc5 was specifically deleted in excitatory or inhibitory neurons in the mouse brain to determine neuronal subtypes that drive epileptogenesis and SUDEP. Electroencephalogram (EEG), cardiac, and respiratory recordings were performed to determine cardiorespiratory phenotypes associated with SUDEP. Baseline respiratory function and the response to hypoxia challenge were also studied in these mice.
Results
Depdc5 deletion in excitatory neurons in cortical layer 5 and dentate gyrus caused frequent generalized tonic–clonic seizures and SUDEP in young adult mice, but Depdc5 deletion in cortical interneurons did not. EEG suppression immediately following ictal offset was observed in fatal and non-fatal seizures, but low amplitude rhythmic theta frequency activity was lost only in fatal seizures. In addition, these mice developed baseline respiratory dysfunction prior to SUDEP, during which ictal apnea occurred long before terminal cardiac asystole.
Interpretation
Depdc5 deletion in excitatory neurons is sufficient to cause DEPDC5-related epilepsy and SUDEP. Ictal apnea and respiratory dysregulation play critical roles in SUDEP. Our study also provides a novel mouse model to investigate the underlying mechanisms of DEPDC5-related epilepsy and SUDEP. ANN NEUROL 2023