2023-11-15 スイス連邦工科大学ローザンヌ校(EPFL)
◆この発見は、治療法の設計の手がかりを提供し、他のウイルスも同様の宿主の防御を利用する可能性を示唆しています。さらに、ウイルスが利用する細胞応答が様々なストレスに対する一般的な反応である可能性も示されました。
<関連情報>
- https://actu.epfl.ch/news/how-the-covid-19-virus-makes-itself-more-infectiou/
- https://www.nature.com/articles/s41467-023-43027-2
SARS-CoV-2は細胞損傷反応を乗っ取り、より効率的なスパイクS-アシルトランスフェラーゼの転写を誘導する SARS-CoV-2 hijacks a cell damage response, which induces transcription of a more efficient Spike S-acyltransferase
Francisco S. Mesquita,Laurence Abrami,Lucie Bracq,Nattawadee Panyain,Vincent Mercier,Béatrice Kunz,Audrey Chuat,Joana Carlevaro-Fita,Didier Trono & F. Gisou van der Goot
Nature Communications Published:11 November 2023
DOI:https://doi.org/10.1038/s41467-023-43027-2
Abstract
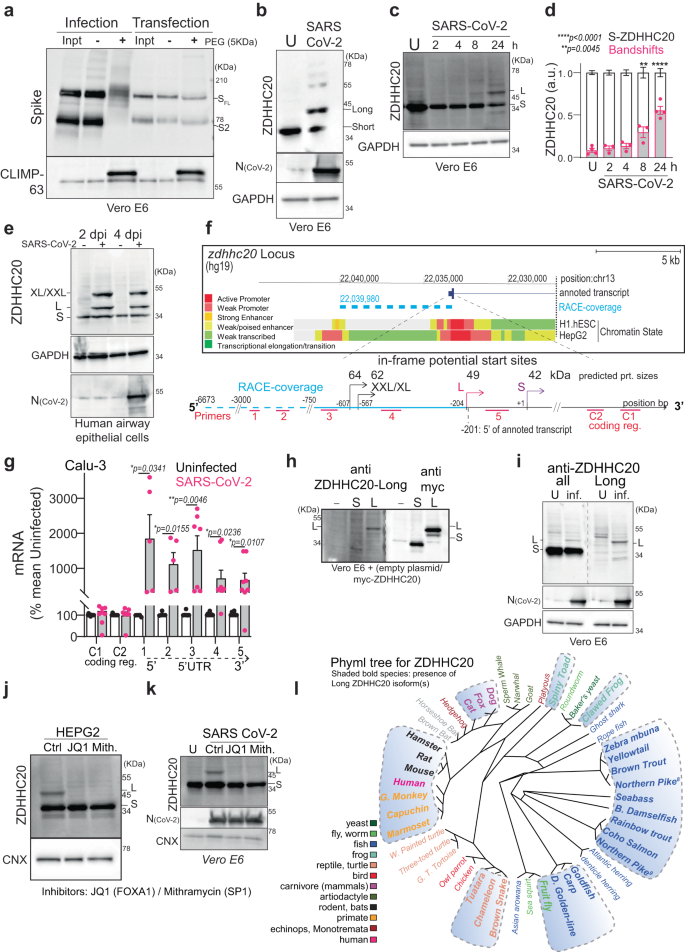
SARS-CoV-2 infection requires Spike protein-mediated fusion between the viral and cellular membranes. The fusogenic activity of Spike depends on its post-translational lipid modification by host S-acyltransferases, predominantly ZDHHC20. Previous observations indicate that SARS-CoV-2 infection augments the S-acylation of Spike when compared to mere Spike transfection. Here, we find that SARS-CoV-2 infection triggers a change in the transcriptional start site of the zdhhc20 gene, both in cells and in an in vivo infection model, resulting in a 67-amino–acid-long N-terminally extended protein with approx. 40 times higher Spike acylating activity, resulting in enhanced fusion of viruses with host cells. Furthermore, we observed the same induced transcriptional change in response to other challenges, such as chemically induced colitis and pore-forming toxins, indicating that SARS-CoV-2 hijacks an existing cell damage response pathway to optimize it fusion glycoprotein.