2025-03-18 ペンシルベニア州立大学 (Penn State)
<関連情報>
- https://www.psu.edu/news/research/story/fda-approved-dialysis-drug-may-help-fight-against-antimicrobial-resistance
- https://onlinelibrary.wiley.com/doi/10.1002/smll.202407549
- https://elifesciences.org/articles/58147
抗生物質耐性の進化を防ぐ高分子抗抗生物質微粒子 Polymeric Anti-Antibiotic Microparticles to Prevent Antibiotic Resistance Evolution
Roya Koshani, Shang-Lin Yeh, Zeming He, Naveen Narasimhalu, Landon G. vom Steeg, Derek G. Sim, Robert J. Woods, Andrew F. Read, Amir Sheikhi
Small Published: 19 January 2025
DOI:https://doi.org/10.1002/smll.202407549

Abstract
Vancomycin (VAN) and daptomycin (DAP) are among the last-resort antibiotics for treating multidrug-resistant Gram-positive bacterial infections. They are administered intravenously (IV); however, ≈5 – 10% of the total IV dose is released in the gastrointestinal (GI) tract via biliary excretion, driving resistance emergence in commensal Enterococcus faecium (E. faecium) populations. Here, it is reported that sevelamer (SEV), a Food and Drug Administration (FDA)-approved anion-exchange polymeric microparticle, captures anionic DAP within minutes and cationic VAN within hours, inactivating the antibacterial efficacy of DAP and VAN. In vitro SEV-mediated VAN or DAP transient removal is successfully described by a diffusion-adsorption mechanism. In vivo oral SEV treatment effectively prevented VAN resistance enrichment following the VAN treatment of E. faecium-colonized mice. This work shows, for the first time, that the adjuvant SEV therapy prevents antimicrobial resistance in nosocomial pathogens by eliminating off-target antibiotics. It is envisioned that SEV may protect DAP and VAN from resistance development, potentially addressing the long-lasting antimicrobial resistance.
抗生物質と併用することで、日和見感染病原体の抗生物質耐性クローンの増加を防ぐことができる An adjunctive therapy administered with an antibiotic prevents enrichment of antibiotic-resistant clones of a colonizing opportunistic pathogen
Valerie J Morley ,Clare L Kinnear,Derek G Sim,Samantha N Olson,Lindsey M Jackson,Elsa Hansen,Grace A Usher,Scott A Showalter,Manjunath P Pai,Andrew F Read
eLife Published:Dec 1, 2020
DOI:https://doi.org/10.7554/eLife.58147
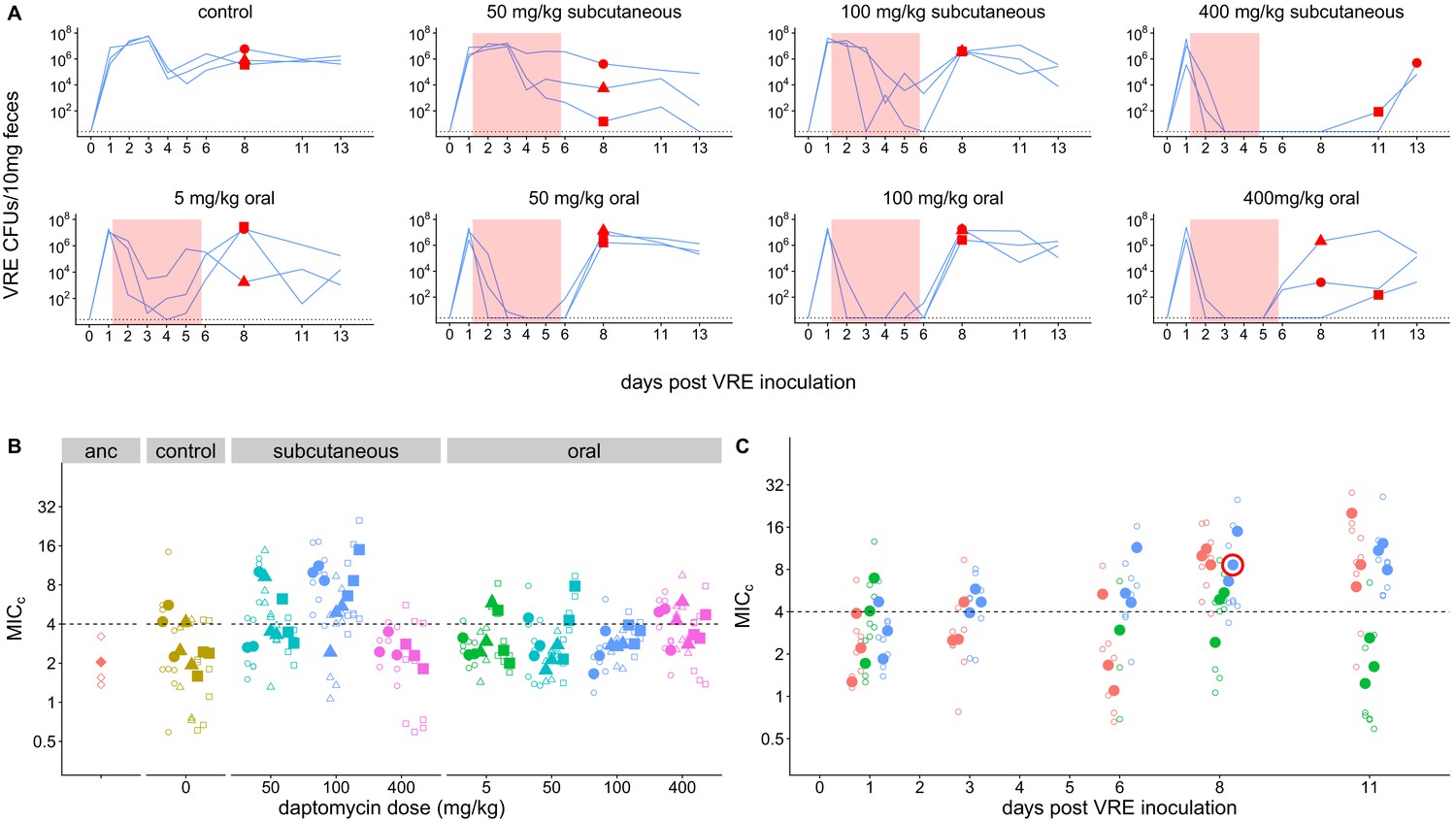
Abstract
A key challenge in antibiotic stewardship is figuring out how to use antibiotics therapeutically without promoting the evolution of antibiotic resistance. Here, we demonstrate proof of concept for an adjunctive therapy that allows intravenous antibiotic treatment without driving the evolution and onward transmission of resistance. We repurposed the FDA-approved bile acid sequestrant cholestyramine, which we show binds the antibiotic daptomycin, as an ‘anti-antibiotic’ to disable systemically-administered daptomycin reaching the gut. We hypothesized that adjunctive cholestyramine could enable therapeutic daptomycin treatment in the bloodstream, while preventing transmissible resistance emergence in opportunistic pathogens colonizing the gastrointestinal tract. We tested this idea in a mouse model of Enterococcus faecium gastrointestinal tract colonization. In mice treated with daptomycin, adjunctive cholestyramine therapy reduced the fecal shedding of daptomycin-resistant E. faecium by up to 80-fold. These results provide proof of concept for an approach that could reduce the spread of antibiotic resistance for important hospital pathogens.


00059-8/asset/e7c3ed66-32f8-4b1e-a052-bd22f609f444/main.assets/gr1_lrg.jpg)