2025-06-24 中国科学院(CAS)
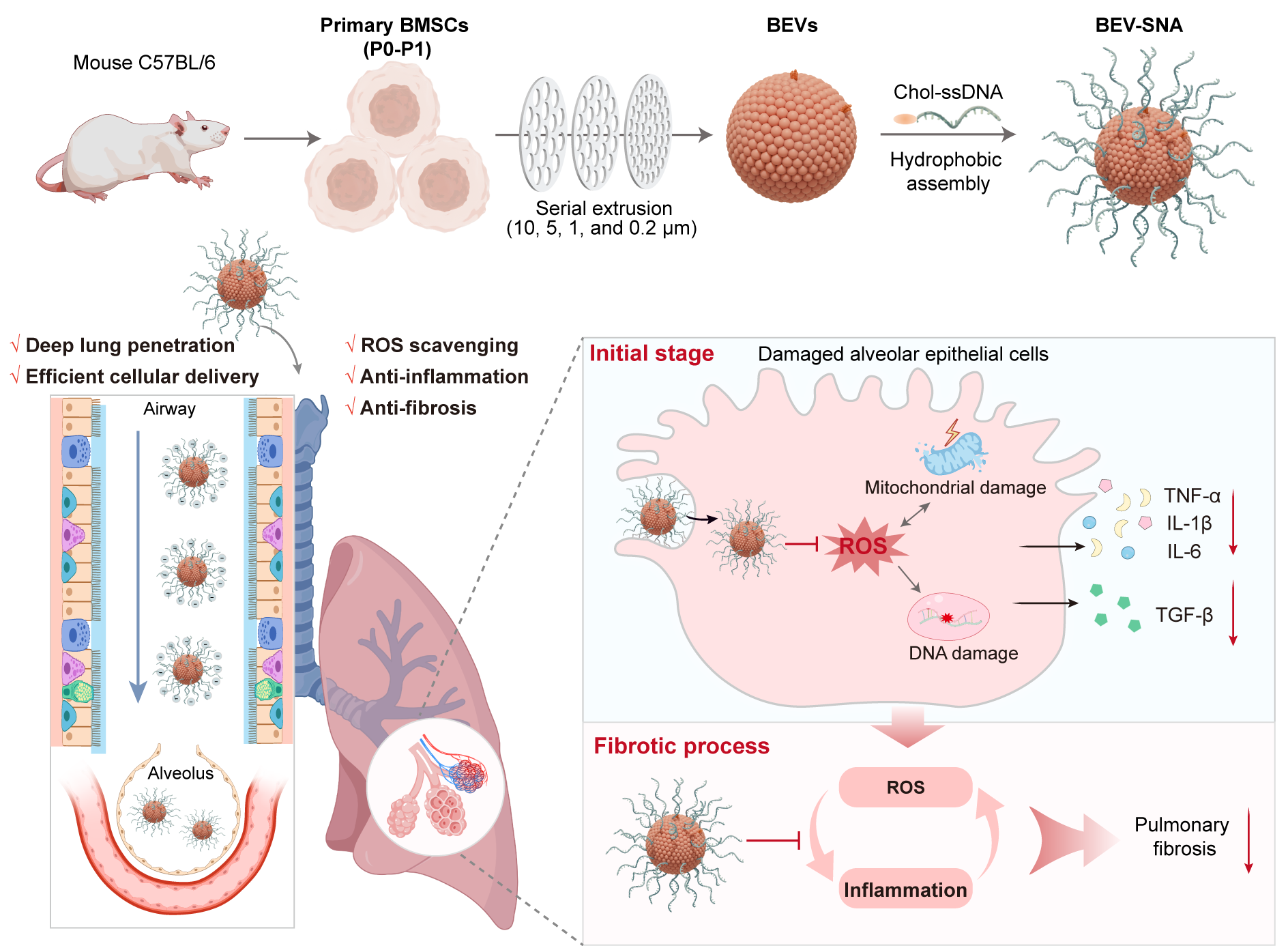 BEV-SNA-mediated therapy for idiopathic pulmonary fibrosis. (Image by NIMTE)
BEV-SNA-mediated therapy for idiopathic pulmonary fibrosis. (Image by NIMTE)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202506/t20250624_1046102.shtml
- https://onlinelibrary.wiley.com/doi/10.1002/agt2.70086
活性酸素消去および抗炎症作用による肺線維症治療のための肺透過性バイオミメティック細胞外小胞球状核酸 Lung-Penetrating Biomimetic Extracellular Vesicle Spherical Nucleic Acids for Pulmonary Fibrosis Therapy Through ROS Scavenging and Anti-Inflammatory Effects
Saiyun Lou, Jiangpo Ma, Pan Fu, Lin Li, Jingyun Huang, Fangxue Jing, Yuhui Wang, Sihua Qian, Jianping Zheng, Jiang Li, Zhaoxing Dong, Kaizhe Wang
Aggregate Published: 12 June 2025
DOI:https://doi.org/10.1002/agt2.70086
ABSTRACT
Idiopathic pulmonary fibrosis (IPF) is an irreversible and fatal lung disease characterized by persistent alveolar epithelial cell injury and extracellular matrix deposition. Early dual modulation of oxidative stress and inflammation may offer a promising therapeutic opportunity. Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) offer therapeutic promise but face challenges in scalability and efficient lung delivery. Here, we developed a biomimetic extracellular vesicle-spherical nucleic acid (BEV-SNA) platform for IPF therapy. BEV-SNA were constructed by integrating mechanically extruded BEVs from primary MSCs with cholesterol-modified ssDNA through hydrophobic co-assembly. In stemness-maintained P0-P1 MSCs, the production of BEVs increased by 17.2-fold compared to natural EVs. Benefiting from a three-dimensionally dense and negatively charged DNA shell, BEV-SNA reduce airway adhesion, enabling deep pulmonary delivery and efficient cellular uptake. In IPF models, BEV-SNA demonstrated multiphase therapeutic effects, including protection of alveolar epithelial cells from ROS, anti-inflammatory activity, and late-stage anti-fibrotic action, effectively halting fibrosis progression and achieving a 50% survival rate in mice. This study presents a novel therapeutic platform combining the natural biomimicry of EVs with the functional adaptability of SNAs, proposing an innovative strategy for pulmonary drug delivery and the treatment of respiratory diseases.