2025-08-04 バース大学
<関連情報>
- https://www.bath.ac.uk/announcements/flawed-advice-on-drug-safety-is-pushing-women-to-stop-breastfeeding-new-study/
- https://internationalbreastfeedingjournal.biomedcentral.com/articles/10.1186/s13006-025-00756-y
薬物使用が授乳継続に与える影響:叙述的統合を伴う系統的レビュー The effect of medication use on breastfeeding continuation: a systematic review with narrative synthesis
Rachel Pilgrim,Mo Kwok,Anthony May,Sarah Chapman & Matthew D. Jones
International Breastfeeding Journal Published:04 August 2025
DOI:https://doi.org/10.1186/s13006-025-00756-y
Abstract
Background
Medication-related breastfeeding discontinuation occurs when women stop breastfeeding due to medication. While many medicines are safe during breastfeeding, women needing medication are less likely to continue. This disconnect may reflect avoidable barriers and missed opportunities for support. This review aimed to determine the proportion of postpartum women needing medication who discontinue breastfeeding, and to identify implicated medications, influencing factors, and risk factors.
Methods
A systematic search of Embase, PubMed, Cochrane Library, PsycINFO, Scopus, and CINAHL was conducted from January 2004 to November 2024. Forward and backward citation searches were performed. Studies from high-income countries with self-reported breastfeeding discontinuation were included. Combination feeding and expressed breastmilk use were permitted. Exclusions included unpublished studies, non-English articles, case studies, HIV-related studies, alternative or illicit medicine use, and women who never initiated breastfeeding. Risk of bias was assessed using validated tools. Narrative synthesis was used to summarize findings.
Results
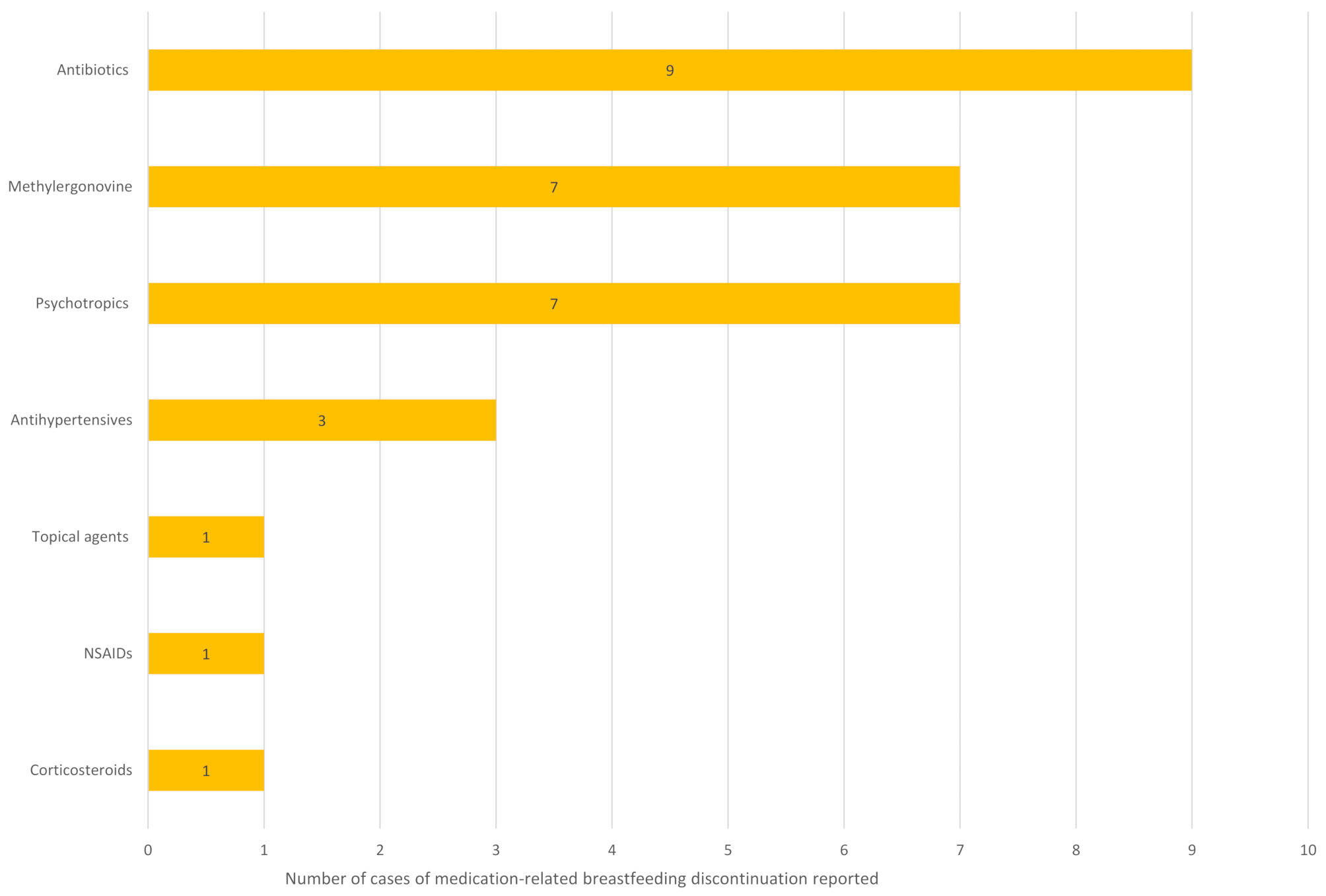
Twenty studies (nine prospective cohort, five retrospective surveys, four qualitative, one randomised controlled trial and one cross-sectional mixed-methods study) were included. Discontinuation rates ranged from 2 to 18% (n = 293) in general populations and 2–58% (n = 1077) in women with chronic or severe acute conditions. Fourteen studies identified 29 medicines involved; all except lithium had post-marketing data indicating safety. Nine studies explored influencing factors. Healthcare professionals were described as encouraging discontinuation in three studies and reducing it in three studies. Other influencing factors were sparsely explored. One study identified risk factors, including lower education, Caesarean section, chronic conditions, employment at six months postpartum, less breastfeeding experience, and pre-pregnancy smoking (p < 0.05). The evidence base was limited by methodological heterogeneity, high bias risk, and low population diversity.
Conclusions
Many instances of medication-related breastfeeding discontinuation may be avoidable, given the safety profiles of most implicated medicines. Inconsistent healthcare advice and systemic barriers likely contribute to unnecessary cessation. Further research should explore sociocultural, psychological, and systemic influences on decision-making, particularly among underrepresented groups, to inform equitable, effective interventions that support both breastfeeding and maternal health.


