2025-08-05 生命創成探究センター

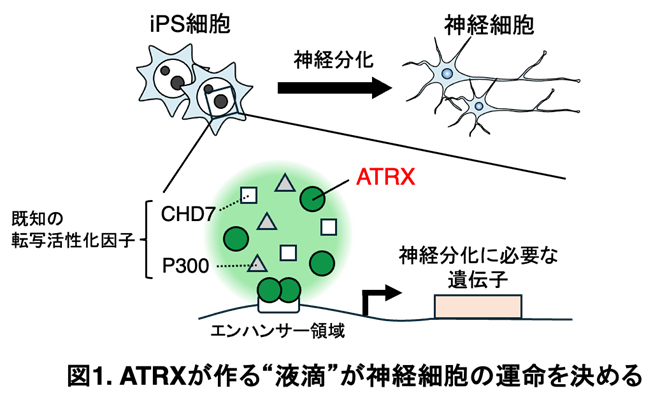
(図)(A)糖鎖のガラクトース残基での構造変化がFc分子内を伝わる様子を示している。(B)ガラクトース残基は糖鎖(黄色の丸)の動きを止める「錨」およびFc領域全体の動きを抑える「楔」としてはたらき、エフェクター分子との相互作用を助けている。
<関連情報>
- https://www.excells.orion.ac.jp/news/12631
- https://www.excells.orion.ac.jp/wp/wp-content/uploads/2025/07/17ba42c86091c36e6ef36270563b27f3.pdf
- https://www.pnas.org/doi/10.1073/pnas.2505473122
計算機シミュレーションと実験的アプローチによるヒト免疫グロブリンGのグリコフォーム依存性動的モジュレーションの探索 Exploring glycoform-dependent dynamic modulations in human immunoglobulin G via computational and experimental approaches
Saeko Yanaka, Yoshitake Sakae, Yohei Miyanoiri, +6 , and Koichi Kato
Proceedings of the National Academy of Sciences Published:August 5, 2025
DOI:https://doi.org/10.1073/pnas.2505473122
Significance
Immunoglobulin G antibodies rely on their Fc region’s interaction with effector molecules to trigger immune responses. We demonstrate how seemingly subtle variations in Fc glycosylation, specifically galactosylation, profoundly impact Fc structure and dynamics. Using a combination of NMR spectroscopy and molecular dynamics simulations, we reveal that galactosylation stabilizes the Fc region, promoting interactions with Fcγ receptors and complement protein C1q. This stabilization is achieved by restricting conformational flexibility. These findings offer a mechanistic understanding of how glycoforms modulate antibody effector functions and provide a rational basis for designing more effective therapeutic antibodies with optimized glycosylation profiles.
Abstract
We investigate the impact of glycoform alterations on the dynamic structure of the human immunoglobulin G1 (IgG1) Fc region using integrated computational and experimental approaches. Four distinct IgG1-Fc glycoforms, varying in core fucosylation and nonreducing terminal galactosylation, were generated through a combination of cell engineering and in vitro enzymatic reactions. Stable-isotope-assisted NMR spectroscopy, incorporating both glycan and protein signals, revealed that galactosylation induces chemical shift perturbations extending from the glycan–protein interface to the CH2–CH3 domain boundary. Molecular dynamics simulations demonstrated that the absence of galactose enhances the mobility of both the glycan and the CH2 domain, broadening the conformational landscape of the Fc quaternary structure. This increased flexibility likely contributes to a greater entropic penalty upon binding to effector molecules, which constrain the Fc in an asymmetric conformation. Conversely, the effects of fucosylation are more localized, primarily influencing the dynamics of residues involved in Fcγ receptor IIIa binding. These findings provide atomic-level insights into the distinct yet synergistic mechanisms by which galactosylation and fucosylation modulate IgG1-Fc dynamics and effector functions, offering crucial information for the optimization of therapeutic antibodies.