2025-08-04 オークリッジ国立研究所(ORNL)
<関連情報>
- https://www.ornl.gov/news/new-imaging-approach-transforms-study-bacterial-biofilms
- https://www.nature.com/articles/s41522-025-00704-y
大面積自動原子間力顕微鏡(AFM)によるバイオフィルム集合体の解析 Analysis of biofilm assembly by large area automated AFM
Ruben Millan-Solsona,Spenser R. Brown,Lance Zhang,Sita Sirisha Madugula,HuanHuan Zhao,Blythe Dumerer,Amber N. Bible,Nickolay V. Lavrik,Rama K. Vasudevan,Arpan Biswas,Jennifer L. Morrell-Falvey,Scott Retterer,Martí Checa & Liam Collins
npj Biofilms and Microbiomes Published:08 May 2025
DOI:https://doi.org/10.1038/s41522-025-00704-y
Abstract
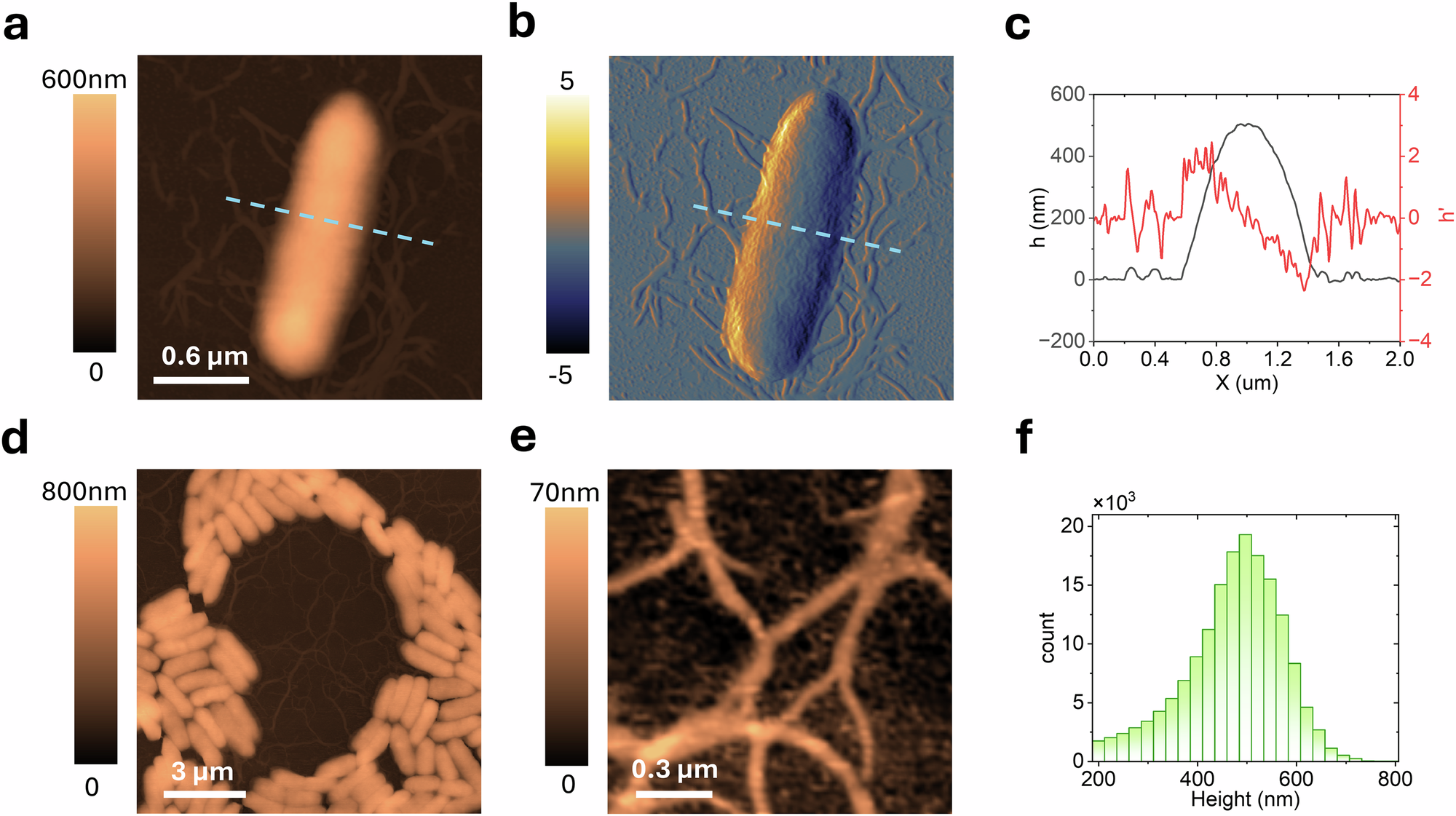
Biofilms are complex microbial communities critical in medical, industrial, and environmental contexts. Understanding their assembly, structure, genetic regulation, interspecies interactions, and environmental responses is key to developing effective control and mitigation strategies. While atomic force microscopy (AFM) offers critically important high-resolution insights on structural and functional properties at the cellular and even sub-cellular level, its limited scan range and labor-intensive nature restricts the ability to link these smaller scale features to the functional macroscale organization of the films. We begin to address this limitation by introducing an automated large area AFM approach capable of capturing high-resolution images over millimeter-scale areas, aided by machine learning for seamless image stitching, cell detection, and classification. Large area AFM is shown to provide a very detailed view of spatial heterogeneity and cellular morphology during the early stages of biofilm formation which were previously obscured. Using this approach, we examined the organization of Pantoea sp. YR343 on PFOTS-treated glass surfaces. Our findings reveal a preferred cellular orientation among surface-attached cells, forming a distinctive honeycomb pattern. Detailed mapping of flagella interactions suggests that flagellar coordination plays a role in biofilm assembly beyond initial attachment. Additionally, we use large-area AFM to characterize surface modifications on silicon substrates, observing a significant reduction in bacterial density. This highlights the potential of this method for studying surface modifications to better understand and control bacterial adhesion and biofilm formation.


