2025-08-28 オックスフォード大学
<関連情報>
- https://www.ox.ac.uk/news/2025-08-28-pressure-flips-switch-cancer-cells
- https://www.nature.com/articles/s41586-025-09445-6
機械的拘束がメラノーマの表現型可塑性を支配する Mechanical confinement governs phenotypic plasticity in melanoma
Miranda V. Hunter,Eshita Joshi,Sydney Bowker,Emily Montal,Yilun Ma,Young Hun Kim,Zhifan Yang,Laura Tuffery,Zhuoning Li,Eric Rosiek,Alexander Browning,Reuben Moncada,Itai Yanai,Helen Byrne,Mara Monetti,Elisa de Stanchina,Pierre-Jacques Hamard,Richard P. Koche & Richard M. White
Nature Published:27 August 2025
DOI:https://doi.org/10.1038/s41586-025-09445-6
Abstract
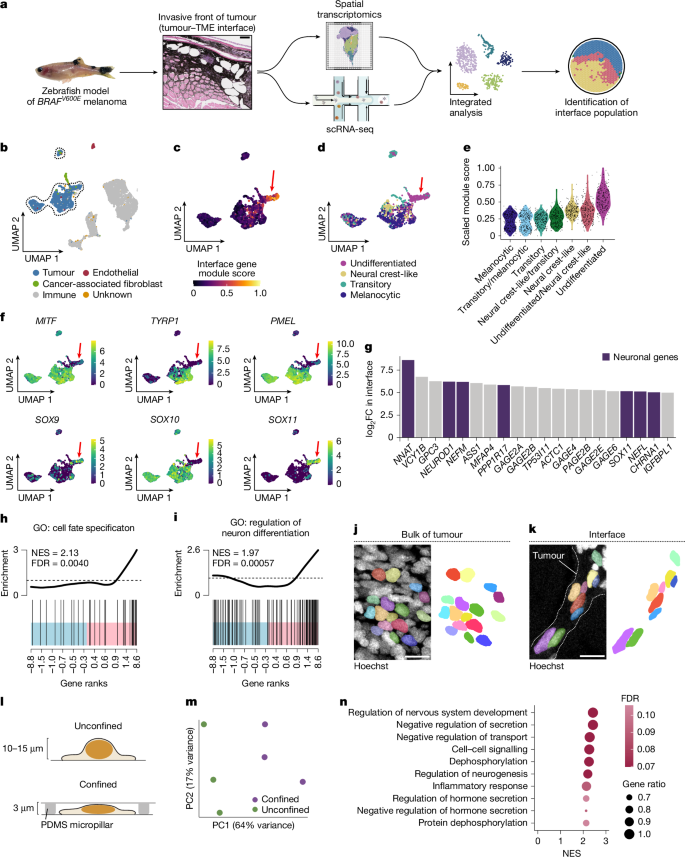
Phenotype switching is a form of cellular plasticity in which cancer cells reversibly move between two opposite extremes: proliferative versus invasive states1,2. Although it has long been hypothesized that such switching is triggered by external cues, the identity of these cues remains unclear. Here we demonstrate that mechanical confinement mediates phenotype switching through chromatin remodelling. Using a zebrafish model of melanoma coupled with human samples, we profiled tumour cells at the interface between the tumour and surrounding microenvironment. Morphological analysis of interface cells showed elliptical nuclei, suggestive of mechanical confinement by the adjacent tissue. Spatial and single-cell transcriptomics demonstrated that interface cells adopted a gene program of neuronal invasion, including the acquisition of an acetylated tubulin cage that protects the nucleus during migration. We identified the DNA-bending protein HMGB2 as a confinement-induced mediator of the neuronal state. HMGB2 is upregulated in confined cells, and quantitative modelling revealed that confinement prolongs the contact time between HMGB2 and chromatin, leading to changes in chromatin configuration that favour the neuronal phenotype. Genetic disruption of HMGB2 showed that it regulates the trade-off between proliferative and invasive states, in which confined HMGB2high tumour cells are less proliferative but more drug-resistant. Our results implicate the mechanical microenvironment as a mechanism that drives phenotype switching in melanoma.


