2024-02-27 マサチューセッツ大学アマースト校
<関連情報>
- https://www.umass.edu/news/article/umass-amherst-researchers-identify-enzyme-key-training-cells-fight-autoimmune
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1292049/full
PRMT5がIL-12治療に対するTh1様iTregsのエピジェネティック変化を制御することを発見
PRMT5 regulates epigenetic changes in suppressive Th1-like iTregs in response to IL-12 treatment
Nidhi Jadon,Sudarvili Shanthalingam,Gregory N. Tew,Lisa M. Minter
Frontiers in Immunology Published:08 January 2024
DOI:https://doi.org/10.3389/fimmu.2023.1292049
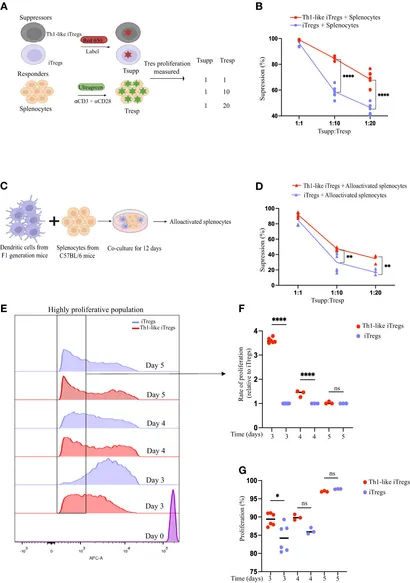
Background: Induced regulatory T cells (iTregs) are a heterogeneous population of immunosuppressive T cells with therapeutic potential. Treg cells show a range of plasticity and can acquire T effector-like capacities, as is the case for T helper 1 (Th1)-like iTregs. Thus, it is important to distinguish between functional plasticity and lineage instability. Aplastic anemia (AA) is an autoimmune disorder characterized by immune-mediated destruction of hematopoietic stem and progenitor cells in the bone marrow (BM). Th1-like 1 iTregs can be potent suppressors of aberrant Th1-mediated immune responses such as those that drive AA disease progression. Here we investigated the function of the epigenetic enzyme, protein arginine methyltransferase 5 (PRMT5), its regulation of the iTreg-destabilizing deacetylase, sirtuin 1 (Sirt1) in suppressive Th1-like iTregs, and the potential for administering Th1-like iTregs as a cell-based therapy for AA.
Methods: We generated Th1-like iTregs by culturing iTregs with IL-12, then assessed their suppressive capacity, expression of iTreg suppression markers, and enzymatic activity of PRMT5 using histone symmetric arginine di-methylation (H3R2me2s) as a read out. We used ChIP sequencing on Th1 cells, iTregs, and Th1-like iTregs to identify H3R2me2s-bound genes unique to Th1-like iTregs, then validated targets using CHiP-qPCR. We knocked down PRMT5 to validate its contribution to Th1-like iTreg lineage commitment. Finally we tested the therapeutic potential of Th1-like iTregs using a Th1-mediated mouse model of AA.
Results: Exposing iTregs to the Th1 cytokine, interleukin-12 (IL-12), during early events of differentiation conveyed increased suppressive function. We observed increased PRMT5 enzymatic activity, as measured by H3R2me2s, in Th1-like iTregs, which was downregulated in iTregs. Using ChIP-sequencing we discovered that H3R2me2s is abundantly bound to the Sirt1 promoter region in Th1-like iTregs to negatively regulate its expression. Furthermore, administering Th1-like iTregs to AA mice provided a survival benefit.
Conclusions: Knocking down PRMT5 in Th1-like iTregs concomitantly reduced their suppressive capacity, supporting the notion that PRMT5 is important for the superior suppressive capacity and stability of Th1-like iTregs. Conclusively, therapeutic administration of Th1-like iTregs in a mouse model of AA significantly extended their survival and they may have therapeutic potential.