2025-09-02 オックスフォード大学
<関連情報>
- https://www.ox.ac.uk/news/2025-09-02-major-new-study-aims-improve-surgery-options-acid-reflux-patients
- https://academic.oup.com/bjs/article/112/7/znaf141/8197872
GOLF試験プロトコル:胃食道逆流症の外科的治療におけるLINX管理システムと胃底折り返し術の比較無作為化臨床試験 Protocol for the GOLF trial: randomized clinical trial on the LINX management system versus fundoplication for the surgical treatment of gastro-oesophageal reflux disease
Sheraz R Markar, Begum Zeybek Saglam, Nainika Menon, Ahmed Ahmed, Nick Maynard, James Gossage, Filipa Landeiro, Jane Blazeby, Nicola Mills, Tim Underwood …
British Journal of Surgery Published:12 July 2025
DOI:https://doi.org/10.1093/bjs/znaf141
Introduction
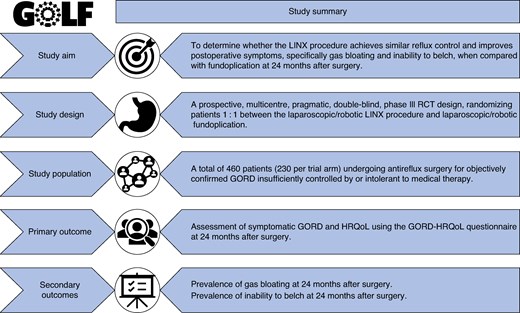
Gastro-oesophageal reflux disease (GORD) represents a significant burden on the Western healthcare system, affecting up to 20% of adults, with a rising prevalence1,2. GORD negatively impacts a patient’s health-related quality of life (HRQoL) and is associated with an increased risk of complications, including inflammation and strictures, Barrett’s oesophagus, and oesophageal adenocarcinoma3. Long-term use of proton pump inhibitors (PPIs) remains the mainstay of medical treatment for GORD; however, these may be associated with an increased risk of side effects, including dementia, renal pathology, infections, fractures, and gastric cancer4. A large UK RCT (REFLUX) showed that surgery (laparoscopic fundoplication) offers the most effective symptom control at 5-year follow-up, as well as being the most cost-effective treatment strategy when compared with medical therapy5,6.
Laparoscopic fundoplication is currently the ‘gold standard’ surgical treatment for managing GORD, with an excellent safety profile and a 30-day mortality risk of 0.03%7. The side effects are mainly gas bloating and inability to belch (up to 85%), dysphagia (3–24%), diarrhoea (18–33%), and recurrence of reflux symptoms (10–62%)8,9. Approximately 5% of patients undergoing fundoplication in England may require secondary surgery and 60% of patients use antireflux medication within 12 months of primary antireflux surgery7.
The introduction of the LINX device in 2007 provided a surgical alternative to fundoplication, requiring less extensive dissection and less disruption of the hiatal anatomy and natural antireflux mechanisms10,11. The LINX device is placed around the distal oesophagus and consists of titanium beads with a magnetic core that augments lower oesophageal tone and thus prevents reflux by mimicking normal anatomical antireflux mechanisms12. The LINX device, while augmenting the lower oesophageal sphincter, can accommodate the escape of elevated gastric pressure associated with belching or vomiting, which may reduce gas bloating. Complications of the LINX device include dysphagia, requiring dilatation at the site of the device in 5–11% of patients, and endoluminal erosions (0.1%) requiring device removal11,13.
In non-randomized comparative studies patients have reported favourable outcomes with LINX compared with fundoplication13. Aside from its ease of insertion, the LINX device is appealing in terms of symptom control, shorter operating time, reduced hospital stay, and lower burden of postoperative care14. A systematic review and meta-analysis of the laparoscopic LINX procedure versus laparoscopic fundoplication (consisting of 6 cohort studies and 1099 patients) showed no statistically significant differences between the groups in the requirement of postoperative antireflux medication, GORD-HRQoL scores, dysphagia, or reoperation. However, LINX was associated with significantly less gas bloating (pooled OR 0.34 (95% c.i. 0.16 to 0.71)) and a greater ability to belch (pooled OR 12.34 (95% c.i. 6.43 to 23.7))13. A further systematic review evaluated the introduction of LINX in the context of the established Idea, Development, Exploration, Assessment, and Long-term follow-up (IDEAL) framework15. Several IDEAL phase IIb studies were identified, with a lack of standardized surgical quality assurance (SQA) regarding LINX implantation and lack of consensus regarding results that should be evaluated to meaningfully assess patient benefit. This review concluded that studies that are well designed and well conducted are needed to evaluate the LINX procedure.
Although the National Institute for Health and Care Excellence (NICE) allows the use of the LINX device in clinical practice, it encourages research in this area, particularly trials that compare the LINX device with other forms of antireflux surgery16. The aim of this RCT is to test the hypothesis that the LINX procedure achieves similar reflux control and improves postoperative symptoms, specifically gas bloating and inability to belch, when compared with fundoplication at 24 months after surgery.

00213-5/asset/b1687a8a-87b6-4c93-8371-fbaf48cc56d0/main.assets/gr1.jpg)