2025-09-11 東京科学大学
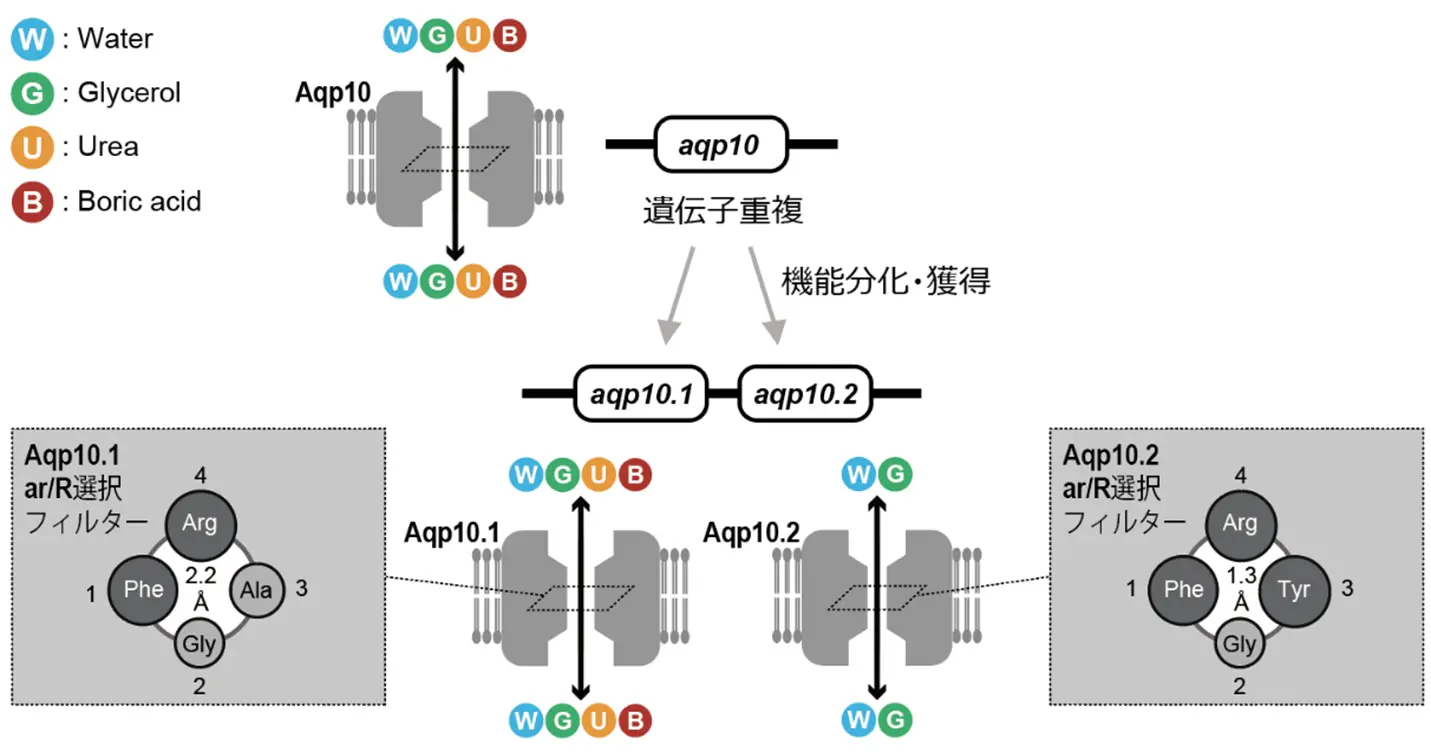
図1. 魚類が遺伝子重複により獲得したAqp10.1とAqp10.2の溶質透過性の違いを生み出すメカニズム。Aqp10.2では、かさ高いアミノ酸残基が細孔のar/R選択フィルターを形成して孔径を狭め、尿素・ホウ酸の透過性を制限することを見出した。
<関連情報>
- https://www.isct.ac.jp/ja/news/fm9ooqv1p1ry
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2226&prevId=&key=ea4387820f48588974a4ca5c74f8e8d5.pdf
- https://journals.physiology.org/doi/full/10.1152/ajpregu.00212.2024
アクアポリン10パラログは、ar/R領域の1番目と3番目のアミノ酸残基に基づく、進化的に変化した尿素およびホウ酸透過性を示す Aquaporin 10 paralogs exhibit evolutionarily altered urea and boric acid permeabilities based on the amino acid residues at positions 1 and 3 in the ar/R region
Ayumi Nagashima,Kazutaka Ushio,Hidenori Nishihara,…
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology
DOI:https://doi.org/10.1152/ajpregu.00212.2024
Abstract
Aquaporin (Aqp)-10 is an aquaglyceroporin permeable to both water and uncharged small-molecule compounds. In ray-finned fish Aqp10 paralogs, urea and boric acid permeabilities of Aqp10.2—but not its glycerol permeability—are much weaker than those of Aqp10.1 and plesiomorphic Aqp10; however, the molecular mechanisms responsible for urea and boric acid permeabilities remain unclear. In this study, we constructed structural models of these sequences and found that two aromatic amino acid residues at positions 1 and 3 of the four amino acid sites in the aromatic/arginine (ar/R) selectivity filter were important in reducing urea and boric acid permeabilities, but not glycerol permeability. Moreover, the characteristics of these amino acid residues could be quantified by calculating the sum of molecular weights of the two amino acid residues. Site-directed mutagenesis revealed that replacement of one of the two aromatic amino acid residues at positions 1 and 3 in the ar/R region with a small amino acid residue enhanced the urea and boric acid permeabilities of Aqp10. In the examined Aqp10s, sum of the molecular weights of amino acid residues at positions 1 and 3 in the ar/R selectivity filter was inversely correlated with the pore diameter and urea and boric acid permeabilities. Overall, our results indicate that the two bulky amino acid residues in the ar/R selectivity filter contribute to the formation of a filter that influences the urea and boric acid permeabilities of aquaglyceroporins.
NEW & NOTEWORTHY Urea and boric acid permeabilities of aquaporin (Aqp)-10.2 are lower than those of Aqp10.1 and plesiomorphic Aqp10, and the molecular weight sum of the two amino acid residues in the aromatic/arginine (ar/R) selectivity filter plays a filtering role that affects permeability. Therefore, urea and boric acid permeabilities of Aqp10s can be assessed using the sum of the molecular weights of the two amino acids in the ar/R region, which represents a significant advancement in this field.