2025-09-12 中国科学院 (CAS)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202509/t20250911_1054398.shtml
- https://www.nature.com/articles/s41586-025-09518-6
近接標識法による抗原誘導性細胞応答の増幅 Amplifying antigen-induced cellular responses with proximity labelling
Shuojun Li,Yinghui Men,Zihan Wang,Yingcheng Wu,Hao Sun,Mingyang Yin,Xinrui Fan,Guiyun Deng,Zhicheng Yang,Tiange Yang,Yudian Xiao,Hu Zhou,Guangchuan Wang,Jia Fan,Chenqi Xu,Qiang Gao & Shuo Han
Nature Published:10 September 2025
DOI:https://doi.org/10.1038/s41586-025-09518-6
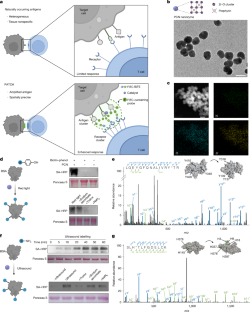
Abstract
Antigen-induced clustering of cell surface receptors, including T cell receptors and Fc receptors, represents a widespread mechanism in cell signalling activation1,2. However, most naturally occurring antigens, such as tumour-associated antigens, stimulate limited receptor clustering and on-target responses owing to insufficient density3,4,5. Here we repurpose proximity labelling6, a method used to biotinylate and identify spatially proximal proteins, to amplify designed probes as synthetic antigen clusters on the cell surface. We develop an in vivo proximity-labelling technology controlled by either red light or ultrasound to covalently tag fluorescein probes at high density near a target antigen. Using T cell receptors as an example, we demonstrate that the amplified fluorescein effectively clusters and directs a fluorescein-binding bispecific T cell engager to induce enhanced T cell activation and cytotoxicity. Noninvasive, tissue-selective labelling in multiple syngeneic mouse tumour models produces potent immune responses that rapidly eradicate treated tumours. Efficient cell lysis further promotes epitope spreading to induce systemic immunity against untreated distal lesions and immune memory against rechallenge. Thus, proximity-labelling chemistry holds promise as a generalized strategy to manipulate antigen-dependent receptor function and cell states.