2025-10-25 千葉大学
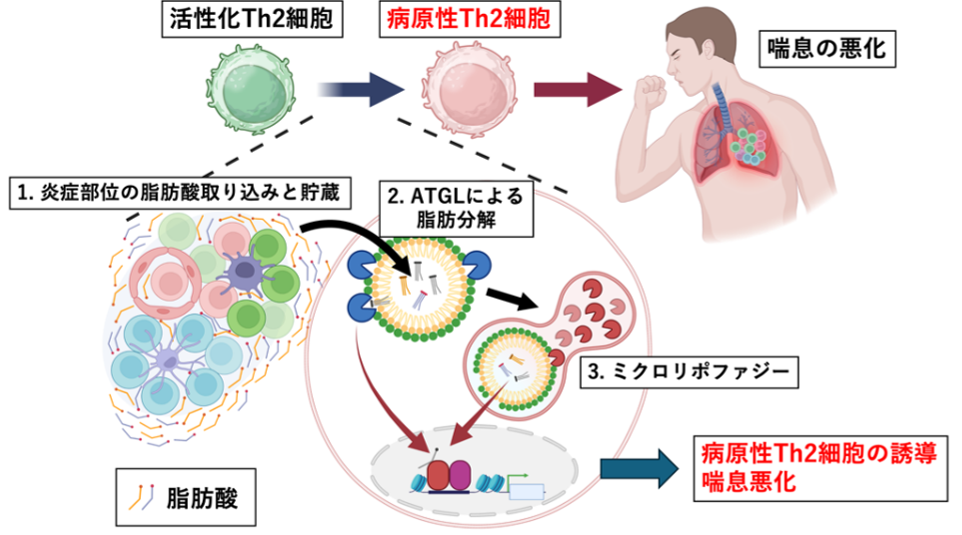
図:脂肪分解経路が病原性Th2細胞を誘導する
<関連情報>
- https://www.chiba-u.ac.jp/news/research-collab/251025_th2.html
- https://www.chiba-u.ac.jp/news/files/pdf/1025_th2.pdf
- https://www.science.org/doi/10.1126/sciimmunol.adp0849
脂肪トリグリセリドリパーゼによって制御される脂肪分解-ミクロリポファジーカスケードは病原性適応型2免疫を駆動する Lipolysis-microlipophagy cascade regulated by adipose triglyceride lipase drives pathogenic adaptive type 2 immunity
Hiroyuki Yagyu, Masahiro Kiuchi, Atsushi Sasaki, Eisuke Itakura, […] , and Kiyoshi Hirahara
Science Immunology Published:24 Oct 2025
DOI:https://doi.org/10.1126/sciimmunol.adp0849
Editor’s summary
Memory T helper 2 (TH2) cells expressing high levels of the interleukin-33 (IL-33) receptor ST2 play an important role in chronic allergic inflammatory diseases. Yagyu et al. report using a mouse model of chronic allergic airway inflammation that activated TH2 cells store long-chain unsaturated fatty acids (LC-UFAs) prevalent in inflamed lungs within lipid droplets (LDs). TH2 cells then catabolize these LDs via adipose triglyceride lipase (ATGL)–mediated lipolysis followed by microlipophagy. LD catabolism in these cells triggers peroxisome proliferator–activated receptor γ (PPARγ), which binds the Il1rl1 locus encoding ST2 and drives the ST2hi TH2 cell phenotype. Patients with eosinophilic chronic rhinosinusitis have memory-type CD4 T cells in their nasal polyps with a similar microlipophagy phenotype. This pathway may, therefore, be an actionable target for treating chronic allergic diseases. —Seth Thomas Scanlon
Abstract
A unique subpopulation of memory T helper 2 (TH2) cells expressing the interleukin-33 (IL-33) receptor ST2 drives allergic disease pathogenesis. However, the immunometabolic mechanisms that induce ST2hi memory TH2 cells remain unclear. We show using a mouse model of chronic allergic airway inflammation that long-chain unsaturated fatty acids (LC-UFAs) accumulate in the inflammatory milieu during chronic airway inflammation. Activated TH2 cells take up LC-UFAs, transiently store them in lipid droplets (LDs), and catabolize LDs through lipolysis and microlipophagy. LD catabolism regulated by adipose triglyceride lipase (ATGL) activates peroxisome proliferator–activated receptor γ (PPARγ). PPARγ then binds the Il1rl1 locus encoding ST2 and induces ST2hi effector and memory TH2 cells. In eosinophilic chronic rhinosinusitis, CD45RO+ CD4 T cells in nasal polyps exhibit microlipophagy and an accessible IL1RL1 enhancer, indicating that these mechanisms are conserved in humans. Thus, the storage and catabolism of inflammatory milieu–derived LC-UFAs direct pathogenic adaptive type 2 immunity, offering potential therapeutic strategies for persistent allergic inflammation.