2025-11-18 カリフォルニア大学サンタバーバラ校(UCSB)
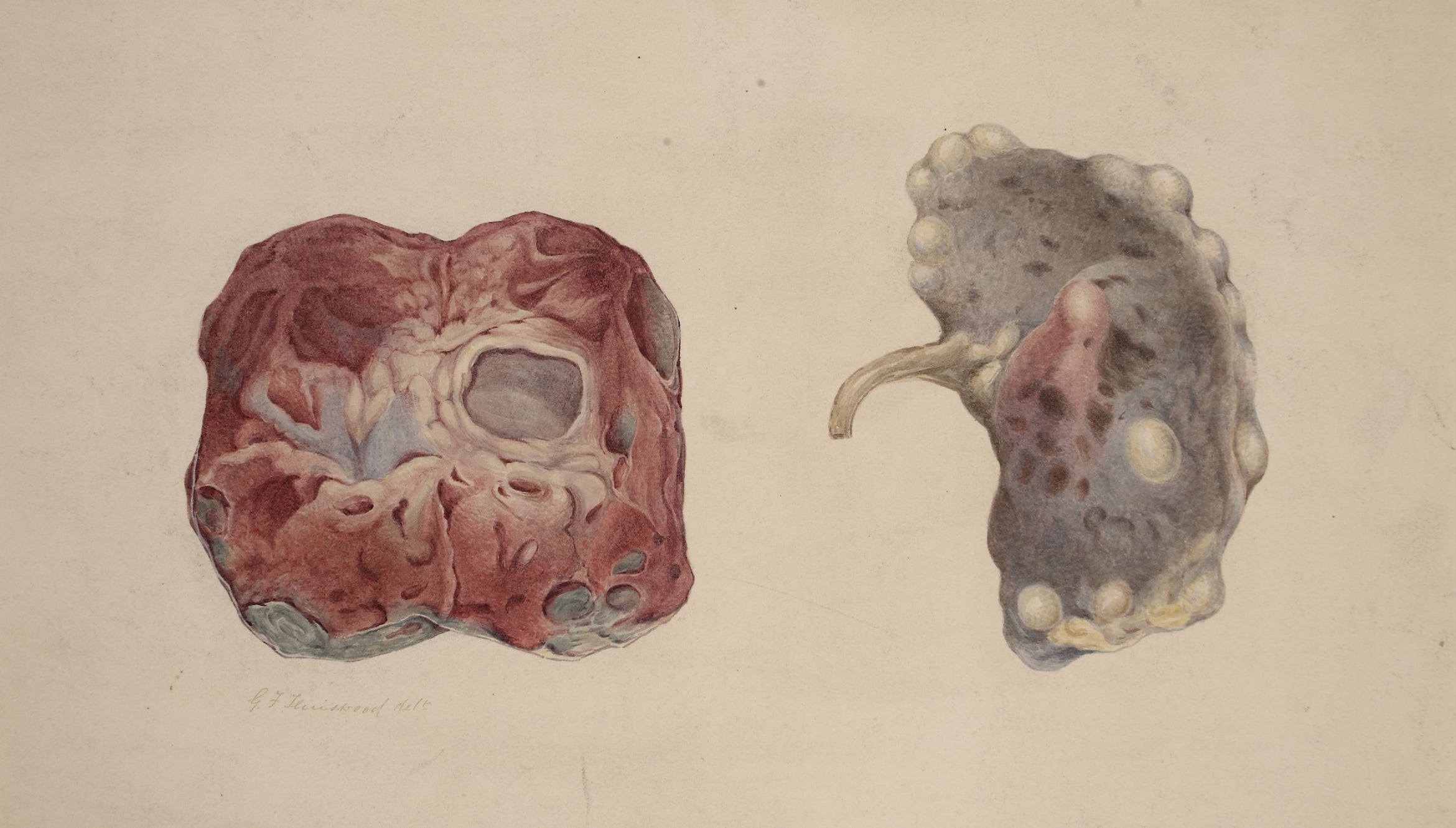
Photo Credit:Teniswood, George Francis, “Polycystic kidney,” Barts Health NHS Trust Archives, c1880-1893 CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons.Watercolor drawing showing two views of a polycystic kidney. One shows the external surface of the kidney, the other when the organ is bisected. Drawing given to the Museum by Dr. Draper Mackinder, MD, Gainsborough, Lincolnshire
<関連情報>
- https://news.ucsb.edu/2025/022244/magic-bullet-polycystic-kidney-disease-making
- https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00408-2
- https://www.sciencedirect.com/science/article/pii/S002192582035064X
cMETに対する拮抗性二量体IgAモノクローナル抗体を用いた多発性嚢胞腎に対する嚢胞標的治療法の開発 Development of a cyst-targeted therapy for polycystic kidney disease using an antagonistic dimeric IgA monoclonal antibody against cMET
Margaret F. Schimmel ∙ Bryan C. Bourgeois ∙ Alison K. Spindt ∙ … ∙ Gavin E. Cornick ∙ Yuqi Liu ∙ Thomas Weimbs
Cell Reports Medicine Published:September 5, 2025
DOI:https://doi.org/10.1016/j.xcrm.2025.102335
Highlights
- Dimeric IgA accumulated specifically in renal cysts at therapeutic concentrations
- cMET-dIgA treatment slowed cyst growth and improved kidney function in PKD mice
- Potent dIgA targeting identified cMET as a key pro-survival factor in PKD cysts
- dIgA offers a versatile platform to repurpose validated mAbs for mucosal diseases
Summary
Polycystic kidney disease (PKD) is characterized by the development of fluid-filled kidney cysts and relentless progression to renal failure. Current treatments have adverse effects and limited efficacy, enhancing the need for improved therapeutics. Here, we provide a proof of concept for the use of dimeric immunoglobulin A (IgA) (dIgA) monoclonal antibodies (mAbs) to target epithelial-enclosed cysts, by exploiting their ability to transcytose via the polymeric immunoglobulin receptor highly expressed on renal cyst-lining cells. We engineered an antagonistic dIgA mAb against the cell mesenchymal-epithelial transition (cMET) receptor, a driver of cyst progression, and demonstrated its specific binding and inhibition of cMET in vitro. In vivo studies in PKD rodent models showed efficient targeting of the mAb to renal cyst lumens and its ability to slow disease progression without apparent adverse effects. This study presents an intriguing avenue for developing antibody-based therapies for PKD and similar diseases by repurposing existing immunoglobulin G (IgG) mAbs into dIgA mAbs for superior targeting to epithelial-enclosed compartments.
多発性嚢胞腎における腎嚢胞腔への抗体標的化のための高分子免疫グロブリン受容体の利用 Exploitation of the Polymeric Immunoglobulin Receptor for Antibody Targeting to Renal Cyst Lumens in Polycystic Kidney Disease
Erin E. Olsan, Tamami Matsushita, Mina Rezaei, Thomas Weimbs
Journal of Biological Chemistry Available online: 28 April 2015
DOI:https://doi.org/10.1074/jbc.M114.607929
Autosomal-dominant polycystic kidney disease (ADPKD) is a common life-threatening genetic disease that leads to renal failure. No treatment is available yet to effectively slow disease progression. Renal cyst growth is, at least in part, driven by the presence of growth factors in the lumens of renal cysts, which are enclosed spaces lacking connections to the tubular system. We have shown previously shown that IL13 in cyst fluid leads to aberrant activation of STAT6 via the IL4/13 receptor. Although antagonistic antibodies against many of the growth factors implicated in ADPKD are already available, they are IgG isotype antibodies that are not expected to gain access to renal cyst lumens. Here we demonstrate that targeting antibodies to renal cyst lumens is possible with the use of dimeric IgA (dIgA) antibodies. Using human ADPKD tissues and polycystic kidney disease mouse models, we show that the polymeric immunoglobulin receptor (pIgR) is highly expressed by renal cyst-lining cells. pIgR expression is, in part, driven by aberrant STAT6 pathway activation. pIgR actively transports dIgA from the circulation across the cyst epithelium and releases it into the cyst lumen as secretory IgA. dIgA administered by intraperitoneal injection is preferentially targeted to polycystic kidneys whereas injected IgG is not. Our results suggest that pIgR-mediated transcytosis of antagonistic antibodies in dIgA format can be exploited for targeted therapy in ADPKD.
Background:
Mitogenic cyst fluid in polycystic kidney disease is not accessible to therapeutic IgG antibodies.
Results:
STAT6 drives expression of the polymeric immunoglobulin receptor, which can transcytose dimeric IgA from the circulation into cyst fluid.
Conclusion:
Dimeric IgA antibodies target to renal cyst lumens.
Significance:
Therapeutic antibodies, reformatted to dIgA, could be evaluated for the treatment of polycystic kidney disease.