2025-12-12 中国科学院(CAS)
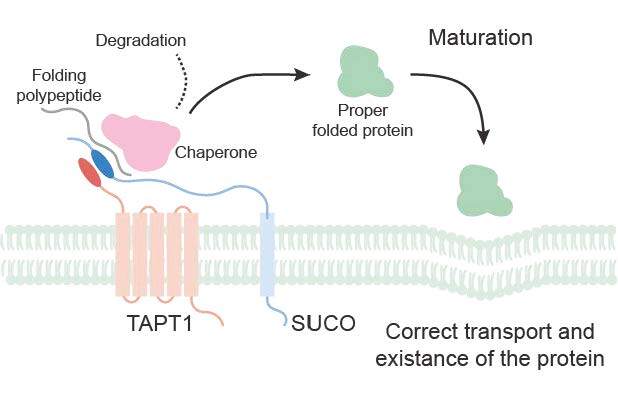
TAPT1 interacts with SUCO to maintain the homeostasis of newly synthesized proteins (Image by XU Zhiheng’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202512/t20251215_1137122.shtml
- https://www.pnas.org/doi/10.1073/pnas.2501361122
TAPT1はSUCOと相互作用して、マウスにおいて新しく合成されたタンパク質の恒常性と脳の発達を維持する TAPT1 interacts with SUCO to maintain the homeostasis of newly synthesized proteins and brain development in mice
Jiawei Gao, Fuqiang Yang, Yisheng Jiang, +6 , and Zhiheng Xu
Proceedings of the National Academy of Sciences Published:December 11, 2025
DOI:https://doi.org/10.1073/pnas.2501361122
Significance
Genetic mutations in TAPT1 cause complex skeletal dysplasia and abnormal brain structure. Here, we demonstrate that Tapt1 knockout in the brain leads to profound neurodevelopmental abnormalities, culminating in severe microcephaly, motor dysfunction and premature death. Significant reductions of many proteins associated with brain development, protein synthesis and transport were observed in Tapt1 knockout brains. Our study shows that transmembrane anterior-posterior transition 1 (TAPT1) interacts with SUN domain-containing ossification factor (SUCO) in the endoplasmic reticulum (ER) to maintain the homeostasis of newly synthesized proteins and is essential for the normal ER-to-Golgi trafficking and organelle structure in human cells and mice. Our findings provide insights into the sharing mechanisms underlying pathogenic TAPT1 and SUCO mutations, offering potential targets for therapeutic alleviation.
Abstract
Genetic mutations in Tapt1 cause complex skeletal dysplasia and structural brain abnormalities. Although the pathogenesis underlying skeletal dysplasia has been explored, the functions and potential mechanisms of transmembrane anterior–posterior transition 1 (TAPT1) during brain development have not been reported. Here, we show that the brains of Tapt1 conditional knockout mice exhibit severe neurodevelopmental defects, including impaired proliferation and differentiation of neural progenitor cells and defects in dendritic and synaptic development, leading to severe microcephaly, motor dysfunction, and early death. Mechanically, we reveal that TAPT1 interacts with SUCO in the endoplasmic reticulum to maintain newly synthesized proteins, including those important for brain development. The TAPT1–SUCO complex plays an essential role in the homeostasis of newly synthesized proteins, and its loss causes overactivated protein degradation, as well as impaired endoplasmic reticulum-to-Golgi trafficking and organelle structures. Our results thus provide insights into the pathogenesis of TAPT1 and SUCO mutation–associated diseases that share similar pathologies.