2025-12-18 国立がん研究センター
<関連情報>
- https://www.ncc.go.jp/jp/information/researchtopics/2025/1218/index.html
- https://www.ncc.go.jp/jp/information/researchtopics/2025/1218/20251218.pdf
- https://www.jto.org/article/S1556-0864(25)02894-1/fulltext
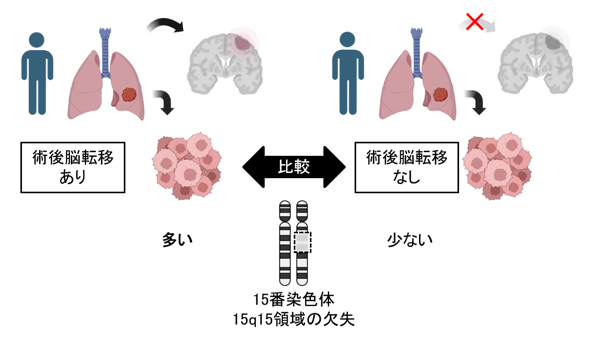
15q15染色体欠失は非小細胞肺癌における脳転移を促進する Chromosome 15q15 Deletion Drives Brain Metastasis in NSCLC
Jun Miyakoshi, MD ∙ Kouya Shiraishi, PhD ∙ Akifumi Mochizuki, MD, PhD ∙ … ∙ Shun-ichi Watanabe, MD, PhD ∙ Ryuji Hamamoto, PhD ∙ Takashi Kohno, PhD
Journal of Thoracic Oncology Published:November 7, 2025
DOI:https://doi.org/10.1016/j.jtho.2025.11.001
Abstract
Introduction
Brain metastasis (BM) is a devastating complication of NSCLC, particularly in NSCLC tumors harboring EGFR mutations. However, the genomic alterations driving BM remain poorly understood.
Methods
We analyzed three independent cohorts of resected NSCLCs from Asian patients, including 1081 primary tumor (PT) samples analyzed by whole-genome sequencing (WGS, discovery cohort, n = 180) or whole-exome sequencing (WES, validation cohort, n = 901), eight BM samples, and 17 matched primary–BM pairs analyzed by WGS (BM cohort). Furthermore, RNA sequencing of the available 172 samples in the validation cohort and drug sensitivity profiling using NSCLC cell lines were performed.
Results
In the discovery cohort, deletions of the 15q15 chromosomal segment were more enriched in PTs from patients with BM than in those without this deletion (73% versus 39%, p = 0.004). Cumulative BM incidence was significantly higher in PTs with the 15q15 deletion (subdistribution hazard ratio = 3.9, p = 0.008 [Fine–Gray competing risk analysis]), whereas metastases at other organ sites did not differ significantly. These findings were obtained using the validation cohort. The 15q15 deletions significantly co-occurred with EGFR mutations (p = 2.8 × 10-7). In matched PT–BM pairs, the 15q15 deletion was detected exclusively in BMs in 46.7% of patients. The deleted region includes the MGA gene, encoding a suppressor of MYC signaling. Transcriptomic analysis revealed MYC signaling and oxidative phosphorylation (OXPHOS) activation in PTs from patients with BM. NSCLC cell lines harboring the 15q15 deletion were selectively sensitive to elesclomol, an OXPHOS inhibitor.
Conclusions
The 15q15 deletion promotes BM development through aberrant MYC signaling and the subsequent reprogramming of carbohydrate metabolism.


