2025-12-11 マサチューセッツ工科大学(MIT)
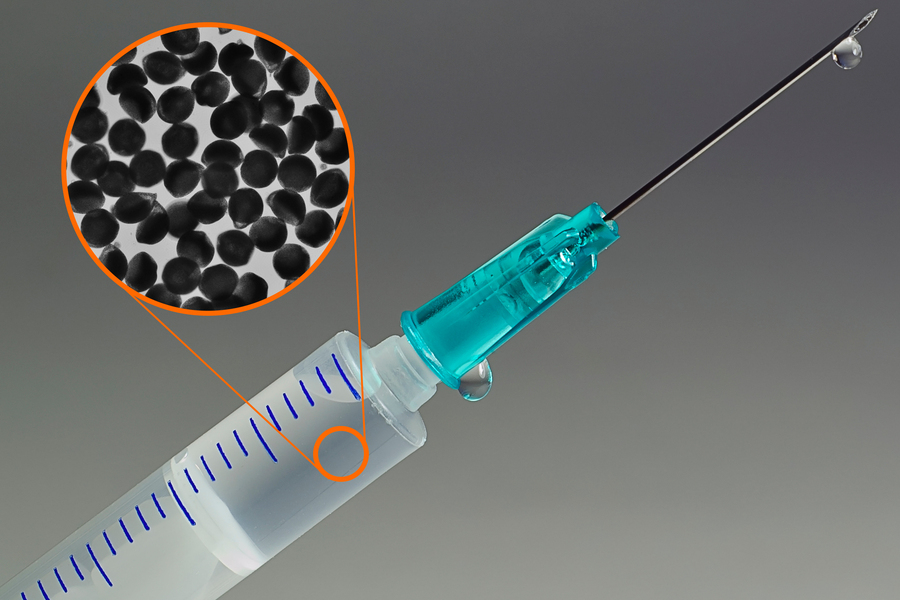
The advance represents a major step toward re-formulating antibodies so that they can be injected using a standard syringe.Credit: Christine Daniloff, MIT; microparticles courtesy of the researchers
<関連情報>
- https://news.mit.edu/2025/new-way-deliver-antibodies-could-make-treatment-much-easier-1211
- https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202516429
溶媒ベースの脱水による高濃度抗体製剤 High-Concentration Antibody Formulation via Solvent-Based Dehydration
Talia Zheng, Lucas Attia, Janet Teng, Patrick S. Doyle
Advanced Materials Published: 23 November 2025
DOI:https://doi.org/10.1002/adma.202516429
Abstract
Although subcutaneous (SC) delivery is the preferred administration route for immunotherapies and other biologics for improved patient compliance and lower healthcare costs, it necessitates high-concentration antibody formulations. However, high-concentration antibody solutions face significant instabilities and prohibitively high viscosities. Other approaches for high-concentration formulations have been developed, including non-aqueous solutions, which can be irritating or painful, and antibody-laden hydrogel microparticles, which require centrifugation and are limited to concentrations <300 mg mL-1. This work presents a new formulation process wherein the antibody is concentrated and encapsulated into hydrogel microparticles via solvent-based dehydration. The final dosage form is an aqueous particle suspension with a formulation concentration of 360 mg mL-1. In this process, microparticles are synthesized continuously, and antibody precipitation is realized simultaneously to dehydration, which allows for higher antibody concentrations. Antibody phase behavior and precipitation–dehydration kinetics are analyzed. The antibody is structurally and functionally stable in the microparticle post-processing and after 4 months. Injectability of the suspension meets clinical standards with glide force <20 N. For the first time, an aqueous antibody formulation at high concentrations comparable to non-aqueous formulations is presented, ideal for subcutaneous administration. The process is envisioned to be generalizable as a platform for SC delivery in multiple clinical applications.

