2026-02-09 カリフォルニア大学サンディエゴ校(UCSD)
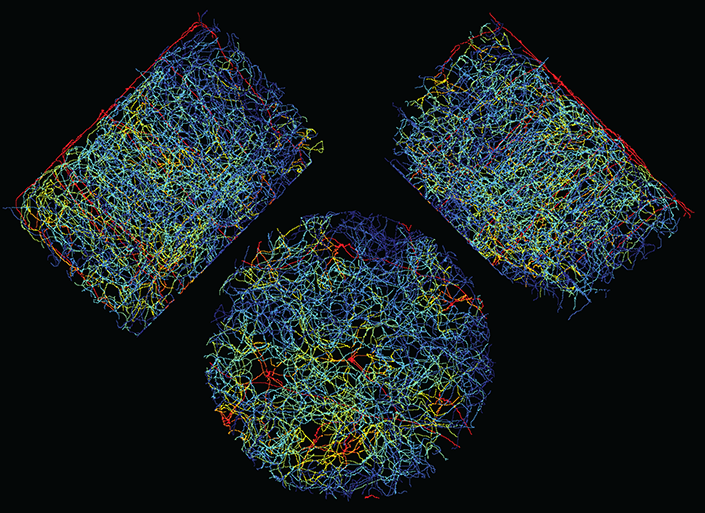
Mouse brain vasculature in a 0.7 mm diameter region of cerebral cortex, labeled to highlight regions of reliable control of blood flow in red. The top-down view and side views are shown.
<関連情報>
- https://today.ucsd.edu/story/how-the-brain-controls-its-blood-flow
- https://www.pnas.org/doi/10.1073/pnas.2521872123
微小血管構造と生理学的変動は脳微小循環の制御を制限する Microvascular architecture and physiological fluctuations constrain the control of cerebral microcirculation
Xiang Ji, Yuchen Zhao, Lu Bai, +1 , and David Kleinfeld
Proceedings of the National Academy of Sciences Published:January 15, 2026
DOI:https://doi.org/10.1073/pnas.2521872123
Significance
The vasculature in the brain forms a multiscale network that actively regulates blood flow to support brain health. To determine how brain angioarchitecture defines physiological limits on the control of cerebral microcirculation, we formulated a theoretical model as well as developed computational tools to analyze data of individual blood cells flowing within cortex. Theory predicts that dilating the entry branch of a diverging node reliably boosts downstream flow and that fluctuations in flow could exhibit both long-range correlations and anticorrelation. The data support these predictions. Notably, diverging nodes preferentially surround penetrating arterioles, which source blood, and are ensheathed by pericytes that enable dilation. More so, brain angioarchitecture necessitates coordinated dilation with explicit constraints to assure the reliable control of flow.
Abstract
Brain vasculature is a multiscale network that actively regulates cerebral blood flow to maintain homeostasis. A systematic understanding of how this network enables robust and precise flow control has been hindered by the lack of understanding of flow in networks, as opposed to single vessels. To address this gap at the conceptual level, we theoretically studied nonperturbative, network-level flow responses to hydrodynamic conductance changes in individual vessels. We show vasodilation can either increase or decrease flow in the neighboring branches, yet selectively positioning the “controller” in the inflow branch of diverging nodes guarantees downstream increases in flow, regardless of surrounding network topology. Moreover, the effect of an individual vasodilation is small, so coordinated vasodilation is essential for effective regulation. To validate and refine our theoretical analysis, we developed a computational framework to analyze individual blood cell motion captured by confocal light field microscopy. This approach enabled tracking over one million cell detections across a network of more than 3,000 interconnected branches, with 2 µm spatial and 14 ms temporal resolution. Network-based analysis uncovered significant flow fluctuations, exhibiting long-range anticorrelation in spatially separated segments. The prevalence of diverging nodes within three branches of penetrating arterioles suggests that ensheathing pericytes are optimally positioned for fine-scale flow regulation. Finally, we quantified a phase separation of blood serum and cells at diverging nodes. This revealed a stochastic partition ratio with a nonlinear dependence on local hemodynamics. Collectively, our work highlights principles of organization for the control of blood flow among the seemingly random connectivity of brain microvessels.


