新開発の小型電極を高血圧対策に活用 Using Newly Developed Mini Electrodes to Fight Hypertension
2022-06-07 ヒューストン大学(UH)
生体医工学のCullen Endowed ProfessorであるMario Romero-Ortegaは、特注の有線電極を用いて、深部腓骨神経刺激(DPNS)が急性血圧の低下を引き起こすことを以前に報告しています。今回の研究は、Frontiers in Neuroscienceに掲載されたもので、小型の埋め込み型無線神経刺激システムの開発と、最大限の低下反応を得るためのさまざまな刺激パラメータの探索を中心に、その研究を進展させました。
サブミリメートル神経刺激回路と、小さな神経への埋め込みを容易にし、外部電源およびDPNS変調制御を可能にする新しい神経取り付けマイクロチャネル電極を統合しました。
この埋め込み型デバイスを用いて、彼のチームは収縮期血圧を神経刺激後1時間で10%、2時間で16%低下させることができることを実証した。
<関連情報>
- https://uh.edu/news-events/stories/2022-news-articles/june-2022/06072022-deep-nerve-stimulation-reduces-blood-pressure-romero-ortega.php
- https://www.frontiersin.org/articles/10.3389/fnins.2022.726467/full
高血圧自然発症ラットにおける深部腓骨神経の神経調節による腎神経活動および動脈圧受容体の変化 Renal Nerve Activity and Arterial Depressor Responses Induced by Neuromodulation of the Deep Peroneal Nerve in Spontaneously Hypertensive Rats
Maria Alejandra Gonzalez-Gonzalez, Kevin Romero, John Beitter, David Lloyd, Danny V. Lam, Ana Guadalupe Hernandez-Reynoso, Aswini Kanneganti, Han-Kyul Kim, Caroline K. Bjune, Scott Smith, Wanpen Vongpatanasin and Mario I. Romero-Ortega
Frontiers in Neuroscience Published:16 May 2022
DOI:https://doi.org/10.3389/fnins.2022.726467
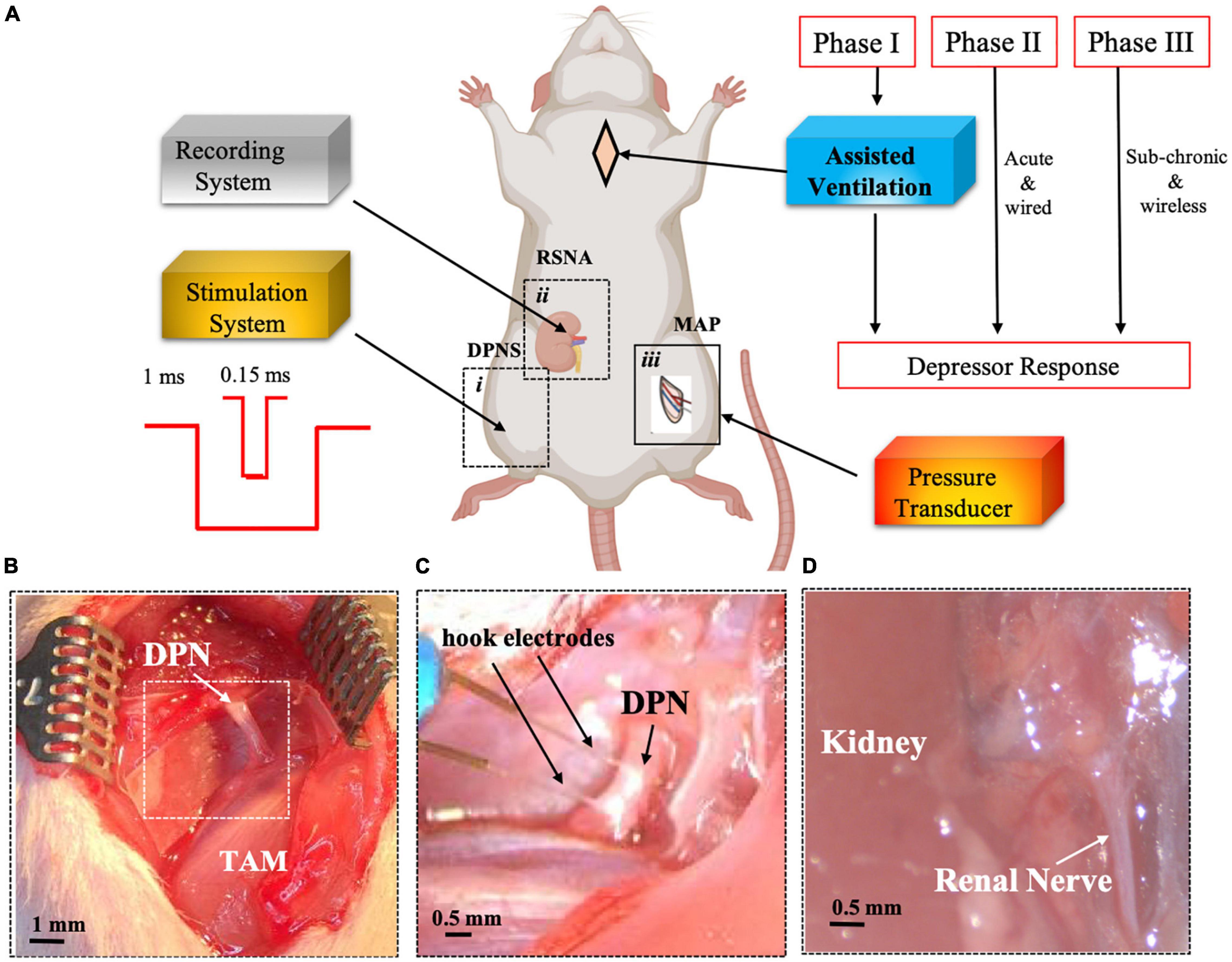
Hypertension is a main cause of death in the United States with more than 103 million adults affected. While pharmacological treatments are effective, blood pressure (BP) remains uncontrolled in 50–60% of resistant hypertensive subjects. Using a custom-wired miniature electrode, we previously reported that deep peroneal nerve stimulation (DPNS) elicited acute cardiovascular depressor responses in anesthetized spontaneously hypertensive rats (SHRs). Here, we further study this effect by implementing a wireless system and exploring different stimulation parameters to achieve a maximum depressor response. Our results indicate that DPNS consistently induces a reduction in BP and suggests that renal sympathetic nerve activity (RSNA) is altered by this bioelectronic treatment. To test the acute effect of DPNS in awake animals, we developed a novel miniaturized wireless microchannel electrode (w-μCE), with a Z-shaped microchannel through which the target nerves slide and lock into the recording/stimulation chamber. Animals implanted with w-μCE and BP telemetry systems for 3 weeks showed an average BP of 150 ± 14 mmHg, which was reduced significantly by an active DPNS session to 135 ± 8 mmHg (p < 0.04), but not in sham-treated animals. The depressor response in animals with an active w-μCE was progressively returned to baseline levels 14 min later (164 ± 26 mmHg). This depressor response was confirmed in restrained fully awake animals that received DPNS for 10 days, where tail-cuff BP measurements showed that systolic BP in SHR lowered 10% at 1 h and 16% 2 h after the DPNS when compared to the post-implantation baseline. Together, these results support the use of DPN neuromodulation as a possible strategy to lower BP in drug-resistant hypertension.