2022-10-18 ペンシルベニア州立大学(PennState)
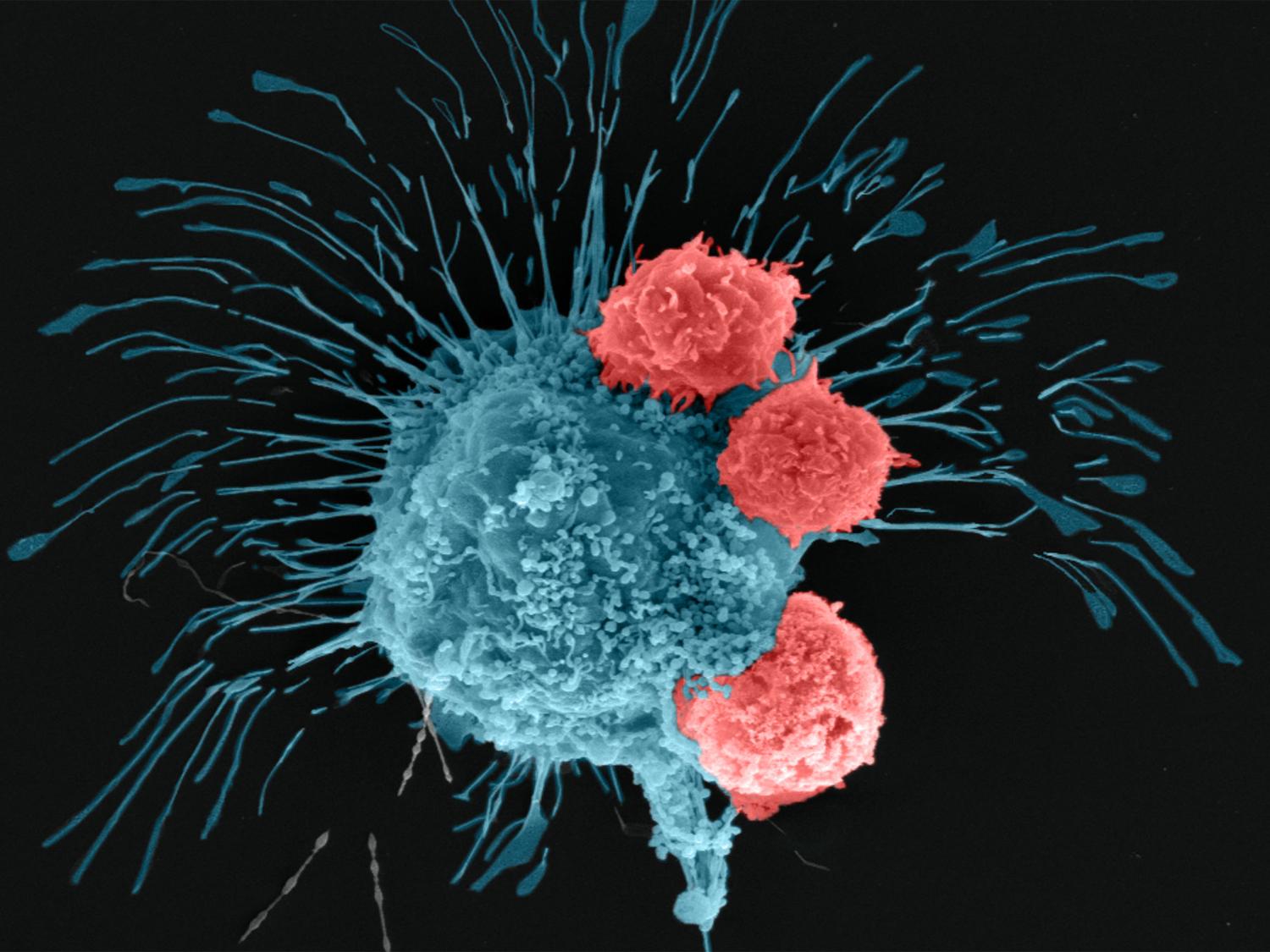
A scanning electron microscopy image shows a breast cancer cell (cyan) being attacked by T cells (red), which were engineered to recognize the cancer cells. Credit: Madhuri Dey, courtesy of the Ozbolat Lab/Penn State. All Rights Reserved.
この技術により、「in vivo」すなわち「動物実験」を行うことなく、抗がん剤治療の研究と開発が可能になる。
研究者らは、吸引支援バイオプリンティングという比較的新しい技術を用いて、腫瘍を3次元的に正確に位置決めし、組織を作製した。その後、この組織を血管のあるマルチスケール乳房腫瘍モデルに形成し、化学療法や細胞ベースの免疫療法に反応することを発見した。
研究チームはまず、乳がんの治療によく用いられるアントラサイクリン系の化学療法剤であるドキソルビシンで腫瘍モデルを処理し、その精度を検証した。
研究者らは、遺伝子編集によって、攻撃的な乳がん細胞を認識して闘うように操作されたヒトCAR-T細胞を使用した。編集したCAR-T細胞を腫瘍内に72時間循環させたところ、バイオプリントされた腫瘍内の細胞が、ポジティブな免疫反応を起こし、がん細胞を撃退していることがわかった。
<関連情報>
- https://www.psu.edu/news/research/story/researchers-3d-bioprint-breast-cancer-tumors-treat-them-groundbreaking-study/
- https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202203966
- https://iopscience.iop.org/article/10.1088/1758-5090/ac925a
3Dバイオプリント血管新生乳がんモデルを用いた化学療法薬およびCAR-T細胞による免疫治療薬スクリーニング Chemotherapeutics and CAR-T Cell-Based Immunotherapeutics Screening on a 3D Bioprinted Vascularized Breast Tumor Model
Madhuri Dey,Myoung Hwan Kim,Mikail Dogan,Momoka Nagamine,Lina Kozhaya,Nazmiye Celik,Derya Unutmaz,Ibrahim T. Ozbolat
Advanced Functional Materials and Biofabrication Published: 03 October 2022
DOI:https://doi.org/10.1002/adfm.202203966
Abstract
Despite substantial advancements in development of cancer treatments, lack of standardized and physiologically-relevant in vitro testing platforms limit the early screening of anticancer agents. A major barrier is the complex interplay between the tumor microenvironment and immune response. To tackle this, a dynamic-flow based 3D bioprinted multi-scale vascularized breast tumor model, responding to chemo and immunotherapeutics is developed. Heterotypic tumors are precisely bioprinted at pre-defined distances from a perfused vasculature, exhibit tumor angiogenesis and cancer cell invasion into the perfused vasculature. Bioprinted tumors treated with varying dosages of doxorubicin for 72 h portray a dose-dependent drug response behavior. More importantly, a cell based immune therapy approach is explored by perfusing HER2-targeting chimeric antigen receptor (CAR) modified CD8+ T cells for 24 or 72 h. Extensive CAR-T cell recruitment to the endothelium, substantial T cell activation and infiltration to the tumor site, resulted in up to ≈70% reduction in tumor volumes. The presented platform paves the way for a robust, precisely fabricated, and physiologically-relevant tumor model for future translation of anti-cancer therapies to personalized medicine.
MAIT細胞受容体を発現するヒトT細胞の細胞傷害応答を解明するための乳がん3次元モデルのバイオファブリケーション Biofabrication of 3D breast cancer models for dissecting the cytotoxic response of human T cells expressing engineered MAIT cell receptors
Madhuri Dey, Myong Hwan Kim, Momoka Nagamine, Ece Karhan, Lina Kozhaya, Mikail Dogan, Derya Unutmaz and Ibrahim T Ozbolat
Biofabrication Published 29 September 2022
DOI:https://doi.org/10.1088/1758-5090/ac925a
Abstract
Immunotherapy has revolutionized cancer treatment with the advent of advanced cell engineering techniques aimed at targeted therapy with reduced systemic toxicity. However, understanding the underlying immune–cancer interactions require development of advanced three-dimensional (3D) models of human tissues. In this study, we fabricated 3D tumor models with increasing complexity to study the cytotoxic responses of CD8+ T cells, genetically engineered to express mucosal-associated invariant T (MAIT) cell receptors, towards MDA-MB-231 breast cancer cells. Homotypic MDA-MB-231 and heterotypic MDA-MB-231/human dermal fibroblast tumor spheroids were primed with precursor MAIT cell ligand 5-amino-6-D-ribitylaminouracil (5-ARU). Engineered T cells effectively eliminated tumors after a 3 d culture period, demonstrating that the engineered T cell receptor recognized major histocompatibility complex class I-related (MR1) protein expressing tumor cells in the presence of 5-ARU. Tumor cell killing efficiency of engineered T cells were also assessed by encapsulating these cells in fibrin, mimicking a tumor extracellular matrix microenvironment. Expression of proinflammatory cytokines such as interferon gamma, interleukin-13, CCL-3 indicated immune cell activation in all tumor models, post immunotherapy. Further, in corroborating the cytotoxic activity, we found that granzymes A and B were also upregulated, in homotypic as well as heterotypic tumors. Finally, a 3D bioprinted tumor model was employed to study the effect of localization of T cells with respect to tumors. T cells bioprinted proximal to the tumor had reduced invasion index and increased cytokine secretion, which indicated a paracrine mode of immune–cancer interaction. Development of 3D tumor-T cell platforms may enable studying the complex immune–cancer interactions and engineering MAIT cells for cell-based cancer immunotherapies.