NHSですでに日常的に行われている遺伝子検査が、腸がん患者の化学療法を導くことができることが、新しい研究で明らかになった。 A genetic test already used routinely in the NHS can guide the use of chemotherapy in people with bowel cancer, new research has shown.
2023-03-02 インペリアル・カレッジ・ロンドン(ICL)
この遺伝子検査は、英国の国民保健サービス(NHS)において既に標準的な治療法として使われており、他のがん治療薬の投与に対する患者の反応を予測するためにも使用されている。この研究により、患者にとって有効ではない場合には不必要な毒性と副作用を避けることができると期待されている。研究者たちは、この結果を早急に指針に取り入れるよう規制当局に呼びかけている。
<関連情報>
- https://www.imperial.ac.uk/news/243473/genetic-test-could-guide-cancer-chemotherapy/
- https://www.nature.com/articles/s41591-023-02240-8
転移性大腸がんにおけるトリフルリジン/チピラシルの生存利益を予測するコドン特異的なKRAS遺伝子変異 Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer
Joris van de Haar,Xuhui Ma,Salo N. Ooft,Pim W. van der Helm,Louisa R. Hoes,Sara Mainardi,David J. Pinato,Kristi Sun,Lisa Salvatore,Giampaolo Tortora,Ina Valeria Zurlo,Silvana Leo,Riccardo Giampieri,Rossana Berardi,Fabio Gelsomino,Valeria Merz,Federica Mazzuca,Lorenzo Antonuzzo,Gerardo Rosati,Chara Stavraka,Paul Ross,Maria Grazia Rodriquenz,Michele Pavarana,Carlo Messina,Timothy Iveson,Federica Zoratto,Anne Thomas,Elisabetta Fenocchio,Margherita Ratti,Ilaria Depetris,Massimiliano Cergnul,Cristina Morelli,Michela Libertini,Alessandro Parisi,Michele De Tursi,Nicoletta Zanaletti,Ornella Garrone,Janet Graham,Raffaella Longarini,Stefania Maria Gobba,Angelica Petrillo,Emiliano Tamburini,Nicla La Verde,Fausto Petrelli,Vincenzo Ricci,Lodewyk F. A. Wessels,Michele Ghidini,Alessio Cortellini,Emile E. Voest & Nicola Valeri
Nature Medicine Published:02 March 2023
DOI:https://doi.org/10.1038/s41591-023-02240-8
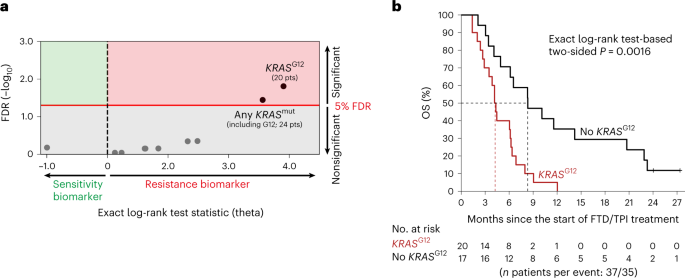
Abstract
Genomics has greatly improved how patients with cancer are being treated; however, clinical-grade genomic biomarkers for chemotherapies are currently lacking. Using whole-genome analysis of 37 patients with metastatic colorectal cancer (mCRC) treated with the chemotherapy trifluridine/tipiracil (FTD/TPI), we identified KRAS codon G12 (KRASG12) mutations as a potential biomarker of resistance. Next, we collected real-world data of 960 patients with mCRC receiving FTD/TPI and validated that KRASG12 mutations were significantly associated with poor survival, also in analyses restricted to the RAS/RAF mutant subgroup. We next analyzed the data of the global, double-blind, placebo-controlled, phase 3 RECOURSE trial (n = 800 patients) and found that KRASG12 mutations (n = 279) were predictive biomarkers for reduced overall survival (OS) benefit of FTD/TPI versus placebo (unadjusted interaction P = 0.0031, adjusted interaction P = 0.015). For patients with KRASG12 mutations in the RECOURSE trial, OS was not prolonged with FTD/TPI versus placebo (n = 279; hazard ratio (HR) = 0.97; 95% confidence interval (CI) = 0.73–1.20; P = 0.85). In contrast, patients with KRASG13 mutant tumors showed significantly improved OS with FTD/TPI versus placebo (n = 60; HR = 0.29; 95% CI = 0.15–0.55; P < 0.001). In isogenic cell lines and patient-derived organoids, KRASG12 mutations were associated with increased resistance to FTD-based genotoxicity. In conclusion, these data show that KRASG12 mutations are biomarkers for reduced OS benefit of FTD/TPI treatment, with potential implications for approximately 28% of patients with mCRC under consideration for treatment with FTD/TPI. Furthermore, our data suggest that genomics-based precision medicine may be possible for a subset of chemotherapies.