2023-03-02 ワシントン大学セントルイス校
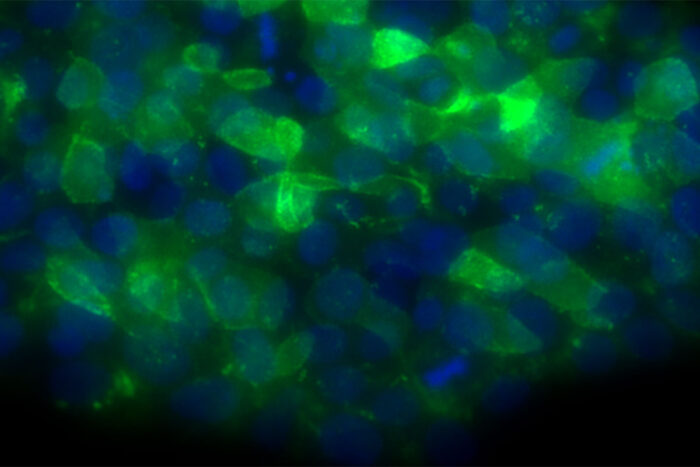
A new study from Washington University School of Medicine in St. Louis identifies a possible treatment strategy for some bone marrow failure syndromes. Shown are human embryonic stem cells engineered to have a mutation that causes poikiloderma with neutropenia, a bone marrow failure syndrome that increases a patient’s risk of developing dangerous infections.
ワシントン大学医学部は、ポイキロデルマと好中球減少症という稀な骨髄不全症候群に対する可能性のある治療戦略を発見したと発表した。
この疾患は、USB1という遺伝子の突然変異によって引き起こされる。この遺伝子異常によって骨髄が機能不全に陥るため、正常な赤血球、白血球、血小板が生成されず、感染症の発生リスクが高くなる。
同校は、この遺伝子の異常に関連する問題を特定し、研究者たちは、PAPD5阻害剤などの薬剤開発に向けて製薬企業と協力している。同疾患を治療するために、PAPD5およびPAPD7阻害剤を特別に設計するための新しい研究が進行中であり、同じような状態を持つ他の骨髄不全症候群にも適用される可能性がある。
<関連情報>
- https://medicine.wustl.edu/news/possible-treatment-strategy-identified-for-bone-marrow-failure-syndrome/
- https://www.science.org/doi/10.1126/science.abj8379
USB1は、造血器官の発達を制御するmiRNA deadenylaseである USB1 is a miRNA deadenylase that regulates hematopoietic development
Ho-Chang Jeong,Siddharth Shukla,Wilson Chun Fok,Thao Ngoc Huynh,Luis Francisco Zirnberger Batista,Roy Parker
Science Published:2 Mar 2023
DOI:https://doi.org/10.1126/science.abj8379
Trimming microRNA for blood development
Mutations in the 3′ to 5′ RNA exonuclease USB1 cause a pediatric disease with defects in the production of blood cells, although the underlying cause of this syndrome is unknown. Jeong et al. determined that USB1 removes extra adenosines from the 3′ end of microRNAs, which if not removed, promote degradation of microRNAs that are necessary for blood development. Blocking the enzyme that adds extra adenosines to microRNAs also restores the production of blood cells in mutant settings. This work determines the molecular basis of disease, identifies new roles for USB1, and suggests a possible therapeutic intervention for patients. —DJ
Abstract
Mutations in the 3′ to 5′ RNA exonuclease USB1 cause hematopoietic failure in poikiloderma with neutropenia (PN). Although USB1 is known to regulate U6 small nuclear RNA maturation, the molecular mechanism underlying PN remains undetermined, as pre-mRNA splicing is unaffected in patients. We generated human embryonic stem cells harboring the PN-associated mutation c.531_delA in USB1 and show that this mutation impairs human hematopoiesis. Dysregulated microRNA (miRNA) levels in USB1 mutants during blood development contribute to hematopoietic failure, because of a failure to remove 3′-end adenylated tails added by PAPD5/7. Modulation of miRNA 3′-end adenylation through genetic or chemical inhibition of PAPD5/7 rescues hematopoiesis in USB1 mutants. This work shows that USB1 acts as a miRNA deadenylase and suggests PAPD5/7 inhibition as a potential therapy for PN.