2023-04-19 リンショーピング大学
この包帯は、密なメッシュ状のナノセルロースで作られており、微生物の侵入を防ぎながら気体や液体を通過させることができます。また、創傷が感染した場合、包帯の色が変化するため、定期的な脱着を行う必要がなく、より効率的な治療が可能となると考えられています。このナノセルロース創傷包帯は、5〜10年以内に医療現場で使用可能となる見込みです。
<関連情報>
- https://liu.se/en/news-item/sarforbandet-som-avslojar-infektion
- https://www.nature.com/articles/s41598-023-31185-8
- https://www.sciencedirect.com/science/article/pii/S2590006423000340
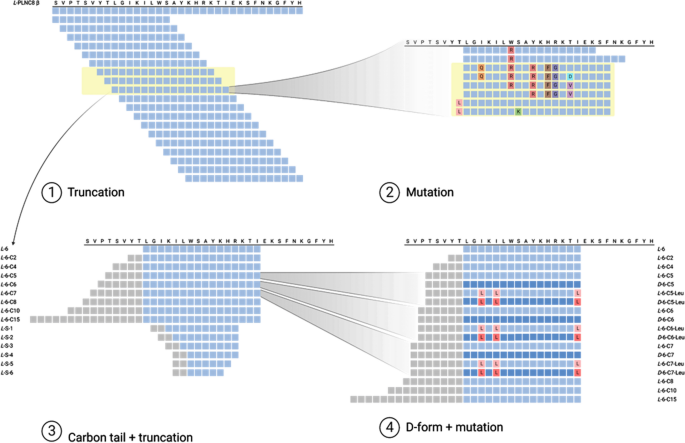
プランタリシンNC8βから誘導される新規広域抗菌リポペプチドの開発 Development of novel broad-spectrum antimicrobial lipopeptides derived from plantaricin NC8 β
Emanuel Wiman,Elisa Zattarin,Daniel Aili,Torbjörn Bengtsson,Robert Selegård & Hazem Khalaf
Scientific Reports Published:13 March 2023
DOI:https://doi.org/10.1038/s41598-023-31185-8
Abstract
Bacterial resistance towards antibiotics is a major global health issue. Very few novel antimicrobial agents and therapies have been made available for clinical use during the past decades, despite an increasing need. Antimicrobial peptides have been intensely studied, many of which have shown great promise in vitro. We have previously demonstrated that the bacteriocin Plantaricin NC8 αβ (PLNC8 αβ) from Lactobacillus plantarum effectively inhibits Staphylococcus spp., and shows little to no cytotoxicity towards human keratinocytes. However, due to its limitations in inhibiting gram-negative species, the aim of the present study was to identify novel antimicrobial peptidomimetic compounds with an enhanced spectrum of activity, derived from the β peptide of PLNC8 αβ. We have rationally designed and synthesized a small library of lipopeptides with significantly improved antimicrobial activity towards both gram-positive and gram-negative bacteria, including the ESKAPE pathogens. The lipopeptides consist of 16 amino acids with a terminal fatty acid chain and assemble into micelles that effectively inhibit and kill bacteria by permeabilizing their cell membranes. They demonstrate low hemolytic activity and liposome model systems further confirm selectivity for bacterial lipid membranes. The combination of lipopeptides with different antibiotics enhanced the effects in a synergistic or additive manner. Our data suggest that the novel lipopeptides are promising as future antimicrobial agents, however additional experiments using relevant animal models are necessary to further validate their in vivo efficacy.
創傷のリアルタイムpHモニタリングのためのナノセルロース複合創傷被覆材 Nanocellulose composite wound dressings for real-time pH wound monitoring
Olof Eskilson, Elisa Zattarin, Linn Berglund, Kristiina Oksman, Kristina Hanna, Jonathan Rakar, Petter Sivlér, Mårten Skog, Ivana Rinklake, Rozalin Shamasha, Zeljana Sotra, Annika Starkenberg, Magnus Odén, Emanuel Wiman, Hazem Khalaf, Torbjörn Bengtsson, Johan P.E. Junker, Robert Selegård, Emma M. Björk, Daniel Aili
Materials Today Bio Available online: 6 February 2023
DOI:https://doi.org/10.1016/j.mtbio.2023.100574

Abstract
The skin is the largest organ of the human body. Wounds disrupt the functions of the skin and can have catastrophic consequences for an individual resulting in significant morbidity and mortality. Wound infections are common and can substantially delay healing and can result in non-healing wounds and sepsis. Early diagnosis and treatment of infection reduce risk of complications and support wound healing. Methods for monitoring of wound pH can facilitate early detection of infection. Here we show a novel strategy for integrating pH sensing capabilities in state-of-the-art hydrogel-based wound dressings fabricated from bacterial nanocellulose (BC). A high surface area material was developed by self-assembly of mesoporous silica nanoparticles (MSNs) in BC. By encapsulating a pH-responsive dye in the MSNs, wound dressings for continuous pH sensing with spatiotemporal resolution were developed. The pH responsive BC-based nanocomposites demonstrated excellent wound dressing properties, with respect to conformability, mechanical properties, and water vapor transmission rate. In addition to facilitating rapid colorimetric assessment of wound pH, this strategy for generating functional BC-MSN nanocomposites can be further be adapted for encapsulation and release of bioactive compounds for treatment of hard-to-heal wounds, enabling development of novel wound care materials.

