2023-08-16 カリフォルニア大学校アーバイン校(UCI)
◆STEP 1試験の結果を基にした予測で、心血管リスク因子も低下。ウェゴビーは肥満患者にとって画期的な治療法であり、リスクとメリットの評価が重要。適切な食事と運動習慣の大切さも強調。
<関連情報>
- https://news.uci.edu/2023/08/16/popular-weight-loss-medication-may-benefit-93-million-u-s-adults-uc-irvine-study-found/
- https://link.springer.com/article/10.1007/s10557-023-07488-3
肥満有病率と心血管疾患イベントに対するセマグルチド治療の米国人口適格性と推定影響 US Population Eligibility and Estimated Impact of Semaglutide Treatment on Obesity Prevalence and Cardiovascular Disease Events
Nathan D. Wong,Hridhay Karthikeyan & Wenjun Fan
Cardiovascular Drugs and Therapy Published: 14 August 2023
DOI:https://doi.org/10.1007/s10557-023-07488-3
Abstract
Background
Semaglutide 2.4 mg benefits weight loss and reduction of cardiovascular disease (CVD) risk factors in adults with obesity. We estimated the US population eligibility for semaglutide 2.4 mg (based on the weight management indication) and the impact on obesity and CVD events.
Methods
We applied STEP 1 trial eligibility criteria to US adults aged ≥ 18 years in the US National Health and Nutrition Examination Survey (NHANES) 2015-2018 to estimate the US eligible population. Semaglutide weight changes in STEP 1 were applied to estimate the population impact on weight changes and obesity prevalence. We also estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. The difference in estimated risks with and without semaglutide “treatment” multiplied by the eligible NHANES weighted population represented the estimated “preventable” CVD events.
Results
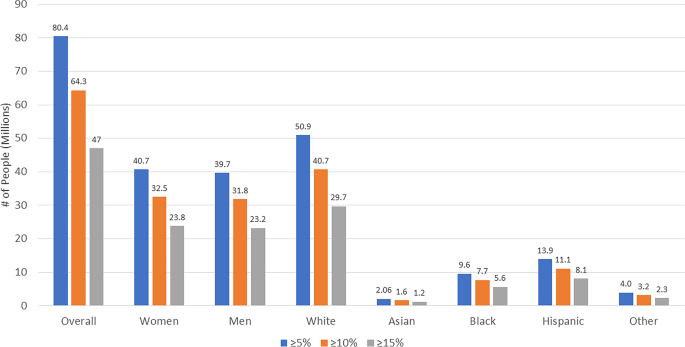
We identified 3999 US adults weighted to an estimated population size of 93.0 million [M] (38% of US adults) who fit STEP 1 eligibility criteria. Applying STEP 1 treatment effects on weight loss resulted in an estimated 69.1% (64.3 M) and 50.5% (47.0 M) showing ≥ 10% and ≥ 15% weight reductions, respectively, translating to a 46.1% (43.0 M) reduction in obesity (BMI ≥ 30 kg/m2) prevalence. Among those without CVD, estimated 10-year CVD risks were 10.15% “before” and 8.34% “after” semaglutide “treatment” reflecting a 1.81% absolute (and 17.8% relative) risk reduction translating to 1.50 million preventable CVD events over 10 years.
Conclusion
Semaglutide treatment in eligible US adults may substantially reduce obesity prevalence and CVD events, which may dramatically impact associated healthcare costs.


