2023-09-08 コロンビア大学
◆この新技術は、遺伝子編集ベクターを脳に送り込み、アルツハイマー病などの脳疾患の原因遺伝子を修正する可能性を提供し、また、アルツハイマー病患者の脳に適用することも可能です。この方法は、脳の免疫システムを活性化し、アルツハイマー病の兆候を改善する効果もあることが示されており、早期段階のアルツハイマー病の治療において重要な役割を果たす可能性があります。
<関連情報>
- https://www.engineering.columbia.edu/news/using-focused-ultrasound-treat-alzheimers-and-parkinsons
- https://www.pnas.org/doi/10.1073/pnas.2302910120
- https://www.thno.org/v13p4102.htm
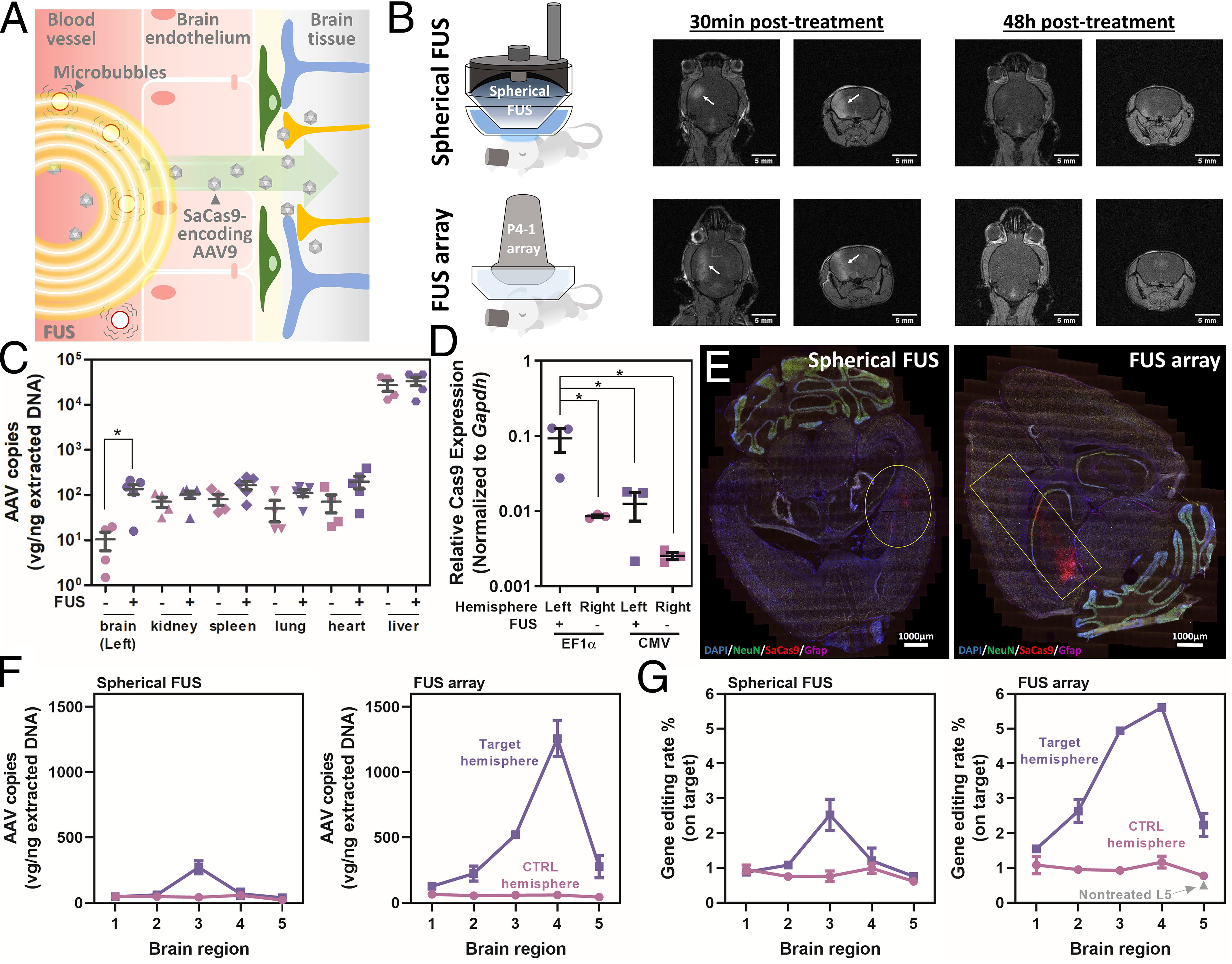
集束超音波を介した脳ゲノム編集 Focused ultrasound–mediated brain genome editing
Yeh-Hsing Lao, Robin Ji , Joyce K. Zhou, Kathy J. Snow , Nancy Kwon , Ethan Saville, Siyu He, Shradha Chauhan, Chun-Wei Chi, Malika S. Datta, Hairong Zhang, Chai Hoon Quek , S. Sarah Cai , Mingqiang Li, Yaned Gaitan , Lawrence Bechtel , Shih-Ying Wu, Cathleen M. Lutz , Raju Tomer, Stephen A. Murray , Alejandro Chavez, Elisa E. Konofagou , and Kam W. Leong
Proceedings of the National Academy of Sciences Published:August 14, 2023
DOI:https://doi.org/10.1073/pnas.2302910120

Abstract
Gene editing in the brain has been challenging because of the restricted transport imposed by the blood–brain barrier (BBB). Current approaches mainly rely on local injection to bypass the BBB. However, such administration is highly invasive and not amenable to treating certain delicate regions of the brain. We demonstrate a safe and effective gene editing technique by using focused ultrasound (FUS) to transiently open the BBB for the transport of intravenously delivered CRISPR/Cas9 machinery to the brain.
集束超音波はアルツハイマー病マウスと患者の病理を緩和し、空間記憶を改善する Focused ultrasound mitigates pathology and improves spatial memory in Alzheimer’s mice and patients
Maria Eleni Karakatsan, Robin Ji, Maria F. Murillo, Tara Kugelman, Nancy Kwon, Yeh-Hsing Lao, Keyu Liu, Antonios N. Pouliopoulos, Lawrence S. Honig, Karen E. Duff, Elisa E. Konofagou
heranostics Published:2023-7-14
DOI:10.7150/thno.79898
Abstract
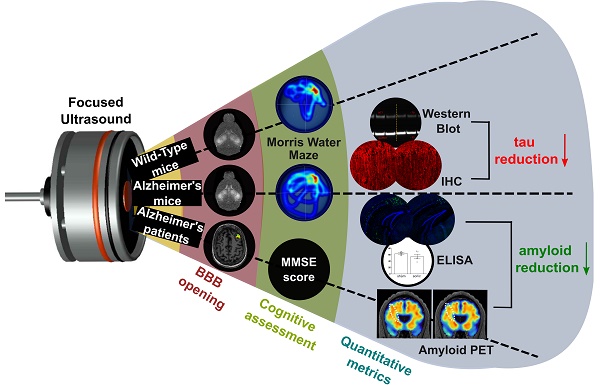
Rationale: Bilateral sonication with focused ultrasound (FUS) in conjunction with microbubbles has been shown to separately reduce amyloid plaques and hyperphosphorylated tau protein in the hippocampal formation and the entorhinal cortex in different mouse models of Alzheimer’s disease (AD) without any therapeutic agents. However, the two pathologies are expressed concurrently in human disease. Therefore, the objective of this study is to investigate the effects of repeated bilateral sonications in the presence of both pathologies.
Methods: Herein, we investigate its functional and morphological outcomes on brains bearing both pathologies simultaneously. Eleven transgenic mice of the 3xTg-AD line (14 months old) expressing human amyloid beta and human tau and eleven age-matched wild-type littermates received four weekly bilateral sonications covering the hippocampus followed by working memory testing. Afterwards, immunohistochemistry and immunoassays (western blot and ELISA) were employed to assess any changes in amyloid beta and human tau. Furthermore, we present preliminary data from our clinical trial using a neuronavigation-guided FUS system for sonications in AD patients (NCT04118764).
Results: Interestingly, both wild-type and transgenic animals that received FUS experienced improved working memory and spent significantly more time in the escape platform-quadrant, with wild-type animals spending 43.2% (sham: 37.7%) and transgenic animals spending 35.3% (sham: 31.0%) of the trial in the target quadrant. Furthermore, this behavioral amelioration in the transgenic animals correlated with a 58.3% decrease in the neuronal length affected by tau and a 27.2% reduction in total tau levels. Amyloid plaque population, volume and overall load were also reduced overall. Consistently, preliminary data from a clinical trial involving AD patients showed a 1.8% decrease of amyloid PET signal 3-weeks after treatment in the treated hemisphere compared to baseline.
Conclusion: For the first time, it is shown that bilateral FUS-induced BBB opening significantly and simultaneously ameliorates both coexistent pathologies, which translated to improvements in spatial memory of transgenic animals with complex AD, the human mimicking phenotype. The level of cognitive improvement was significantly correlated with the volume of BBB opening. Non-transgenic animals were also shown to exhibit similar memory amelioration for the first time, indicating that BBB opening results into benefits in the neuronal function regardless of the existence of AD pathology. A potential mechanism of action for the reduction of the both pathologies investigated was the cholesterol metabolism, specifically the LRP1b receptor, which exhibited increased expression levels in transgenic mice following FUS-induced BBB opening. Initial clinical evidence supported that the beta amyloid reduction shown in rodents could be translatable to humans with significant amyloid reduction shown in the treated hemisphere.