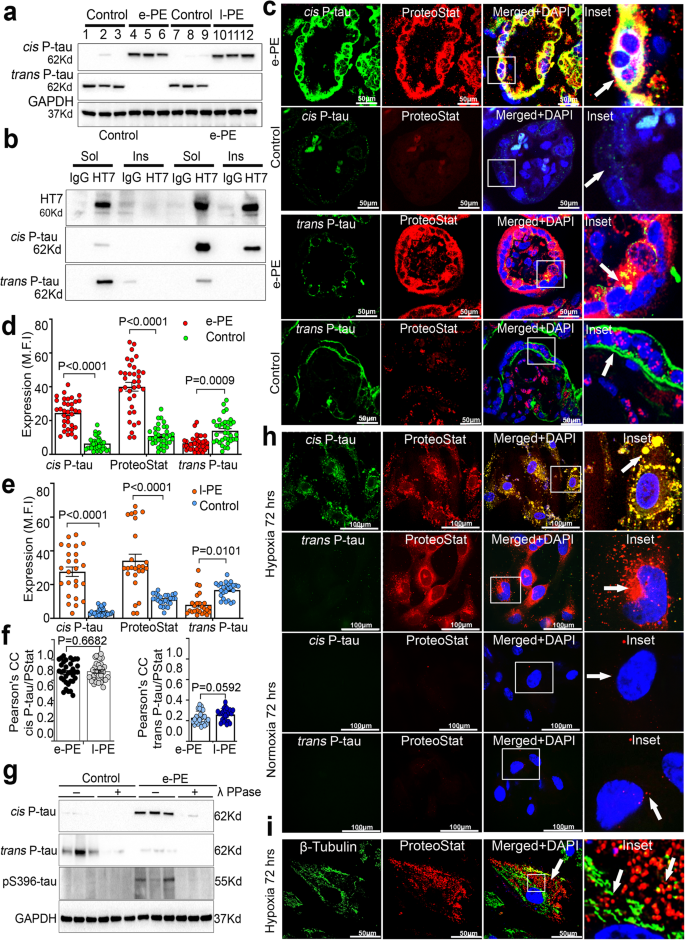
2023-09-11 ミシガン大学
◆このモデルは既存の抗体だけでなく、新しい抗体やまだ作成されていない抗体にも適用可能で、薬の開発を効率化するために活用されています。
<関連情報>
- https://news.umich.edu/ai-tool-helps-optimize-antibody-medicines/
- https://www.nature.com/articles/s41551-023-01074-6
解釈可能な機械学習により、自己会合と非特異的結合を低減する治療用抗体の最適化 Optimization of therapeutic antibodies for reduced self-association and non-specific binding via interpretable machine learning
Emily K. Makowski,Tiexin Wang,Jennifer M. Zupancic,Jie Huang,Lina Wu,John S. Schardt,Anne S. De Groot,Stephanie L. Elkins,William D. Martin & Peter M. Tessier
Nature Biomedical Engineering Published:04 September 2023
DOI:https://doi.org/10.1038/s41551-023-01074-6
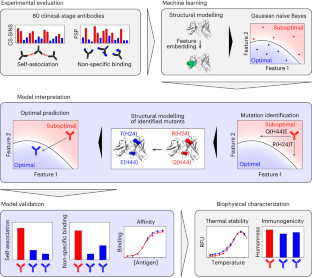
Abstract
Antibody development, delivery, and efficacy are influenced by antibody-antigen affinity interactions, off-target interactions that reduce antibody bioavailability and pharmacokinetics, and repulsive self-interactions that increase the stability of concentrated antibody formulations and reduce their corresponding viscosity. Yet identifying antibody variants with optimal combinations of these three types of interactions is challenging. Here we show that interpretable machine-learning classifiers, leveraging antibody structural features descriptive of their variable regions and trained on experimental data for a panel of 80 clinical-stage monoclonal antibodies, can identify antibodies with optimal combinations of low off-target binding in a common physiological-solution condition and low self-association in a common antibody-formulation condition. For three clinical-stage antibodies with suboptimal combinations of off-target binding and self-association, the classifiers predicted variable-region mutations that optimized non-affinity interactions while maintaining high-affinity antibody-antigen interactions. Interpretable machine-learning models may facilitate the optimization of antibody candidates for therapeutic applications.