2023-10-17 カリフォルニア大学サンディエゴ校(UCSD)
◆研究は、2023年10月17日にeLife誌に掲載されました。細胞の増殖を制御する分子プロセスについての理解は、がん研究や生物医学における基本的な課題であり、肝臓を研究対象にしたこの発見は、がん治療の新しいアプローチを提供する可能性があります。
<関連情報>
- https://today.ucsd.edu/story/new-cancer-therapy-target-stops-tumor-cells-from-sharing-resources
- https://elifesciences.org/articles/86824
細胞内シグナル欠損を相殺する細胞間コミュニケーションにおけるCD133+インターセルソームの同定 Identification of CD133+ intercellsomes in intercellular communication to offset intracellular signal deficit
Kota Kaneko,Yan Liang,Qing Liu,Shuo Zhang,Alexander Scheiter,Dan Song,Gen-Sheng Feng
eLife Published:Oct 17, 2023
DOI:https://doi.org/10.7554/eLife.86824.3
Abstract
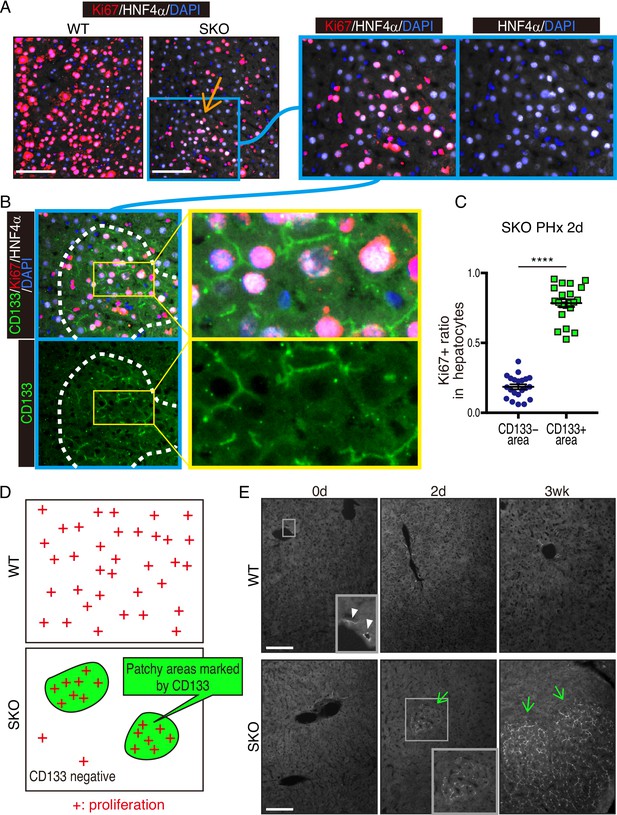
CD133 (prominin 1) is widely viewed as a cancer stem cell marker in association with drug resistance and cancer recurrence. Herein, we report that with impaired RTK-Shp2-Ras-Erk signaling, heterogenous hepatocytes form clusters that manage to divide during mouse liver regeneration. These hepatocytes are characterized by upregulated CD133 while negative for other progenitor cell markers. Pharmaceutical inhibition of proliferative signaling also induced CD133 expression in various cancer cell types from multiple animal species, suggesting an inherent and common mechanism of stress response. Super-resolution and electron microscopy localize CD133 on intracellular vesicles that apparently migrate between cells, which we name ‘intercellsome.’ Isolated CD133+ intercellsomes are enriched with mRNAs rather than miRNAs. Single-cell RNA sequencing reveals lower intracellular diversity (entropy) of mitogenic mRNAs in Shp2-deficient cells, which may be remedied by intercellular mRNA exchanges between CD133+ cells. CD133-deficient cells are more sensitive to proliferative signal inhibition in livers and intestinal organoids. These data suggest a mechanism of intercellular communication to compensate for intracellular signal deficit in various cell types.

