2023-11-20 カリフォルニア大学校アーバイン校(UCI)
◆この発見は、Journal of Neuroinflammationで最近オンラインで発表され、赤血球が脳毛細血管で停滞し、その後どのようにして出血が発生するかを研究チームが観察できるプロセスについて説明しています。脳微小出血は、高血圧、アルツハイマー病、虚血性脳卒中など、高齢者でより頻繁に発生するさまざまな状態と関連しています。
<関連情報>
- https://news.uci.edu/2023/11/20/uc-irvine-led-study-is-first-to-find-brain-hemorrhage-cause-other-than-injured-blood-vessels/
- https://link.springer.com/article/10.1186/s12974-023-02932-5
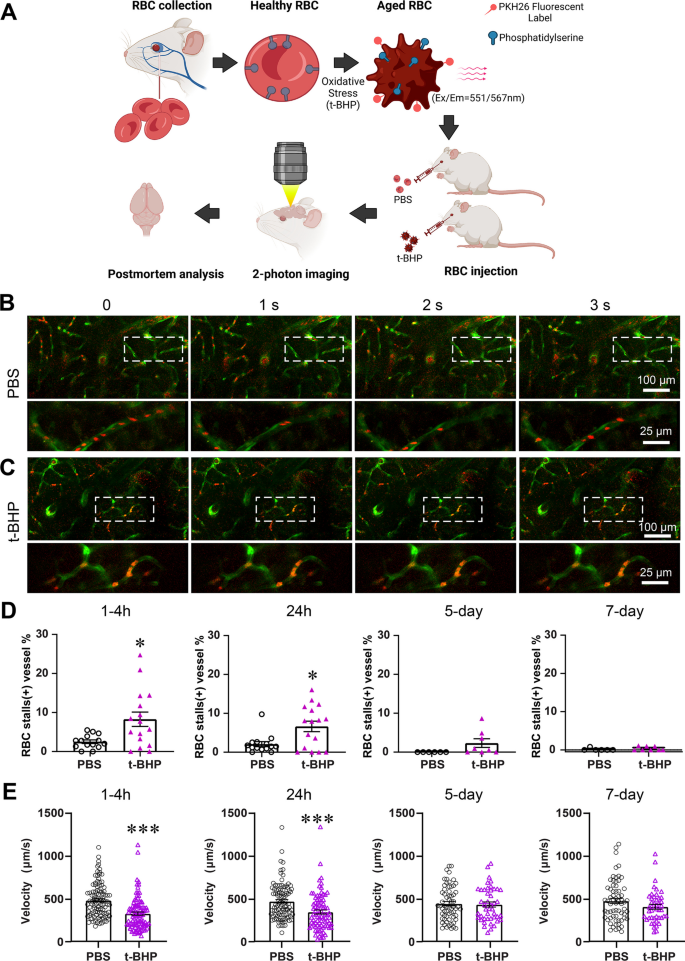
赤血球-脳内皮相互作用はin vivoでミクログリア応答と脳微小出血を誘発する Erythrocyte–brain endothelial interactions induce microglial responses and cerebral microhemorrhages in vivo
Hai Zhang,Rachita K. Sumbria,Rudy Chang,Jiahong Sun,David H. Cribbs,Todd C. Holmes,Mark J. Fisher & Xiangmin Xu
Journal of Neuroinflammation Published: 15 November 2023
DOI:https://doi.org/10.1186/s12974-023-02932-5
Abstract
Background
Cerebral microhemorrhages (CMH) are associated with stroke, cognitive decline, and normal aging. Our previous study shows that the interaction between oxidatively stressed red blood cells (RBC) and cerebral endothelium may underlie CMH development. However, the real-time examination of altered RBC–brain endothelial interactions in vivo, and their relationship with clearance of stalled RBC, microglial responses, and CMH development, has not been reported.
Methods
RBC were oxidatively stressed using tert-butylhydroperoxide (t-BHP), fluorescently labeled and injected into adult Tie2-GFP mice. In vivo two-photon imaging and ex vivo confocal microscopy were used to evaluate the temporal profile of RBC–brain endothelial interactions associated with oxidatively stressed RBC. Their relationship with microglial activation and CMH was examined with post-mortem histology.
Results
Oxidatively stressed RBC stall significantly and rapidly in cerebral vessels in mice, accompanied by decreased blood flow velocity which recovers at 5 days. Post-mortem histology confirms significantly greater RBC–cerebral endothelial interactions and microglial activation at 24 h after t-BHP-treated RBC injection, which persist at 7 days. Furthermore, significant CMH develop in the absence of blood–brain barrier leakage after t-BHP-RBC injection.
Conclusions
Our in vivo and ex vivo findings show the stalling and clearance of oxidatively stressed RBC in cerebral capillaries, highlighting the significance of microglial responses and altered RBC–brain endothelial interactions in CMH development. Our study provides novel mechanistic insight into CMH associated with pathological conditions with increased RBC–brain endothelial interactions.


