2024-01-03 ワシントン大学セントルイス校
◆研究者らは、環境性腸機能障害のある研究の子供たちの多くが、遺伝的に食事性タンパク質をNADに変換するのが困難になる傾向があることを発見しました。食事性タンパク質は、すべての細胞に存在するヘルパー分子であるニコチンアミドアデニンジヌクレオチド(NAD)の供給を生成および維持するために重要です。
◆これにより、栄養介入だけでなく薬物療法も組み合わせることで、従来の治療に反応しない子供たちへの新しいアプローチが可能になるかもしれません。
<関連情報>
- https://source.wustl.edu/2024/01/metabolism-boosting-bile-acid-reducing-drugs-improve-gut-health/
- https://www.science.org/doi/10.1126/scitranslmed.abq4145
NAD+前駆体と胆汁酸減少薬が難治性環境性腸機能障害を治療する NAD+ precursors and bile acid sequestration treat preclinical refractory environmental enteric dysfunction
Atika Malique,Shengxiang Sun,Kanta Chandwe,Beatrice Amadi,Talin Haritunians,Umang Jain,Brian D. Muegge,Jennifer Frein,Yo Sasaki,Amanda Foster,Chad E. Storer,Emebet Mengesha ,Justin Kern,Dermot P.B. McGovern,Richard D. Head,Paul Kelly,and Ta-Chiang Liu
Science Translational Medicine Published:3 Jan 2024
DOI:https://doi.org/10.1126/scitranslmed.abq4145

Editor’s summary
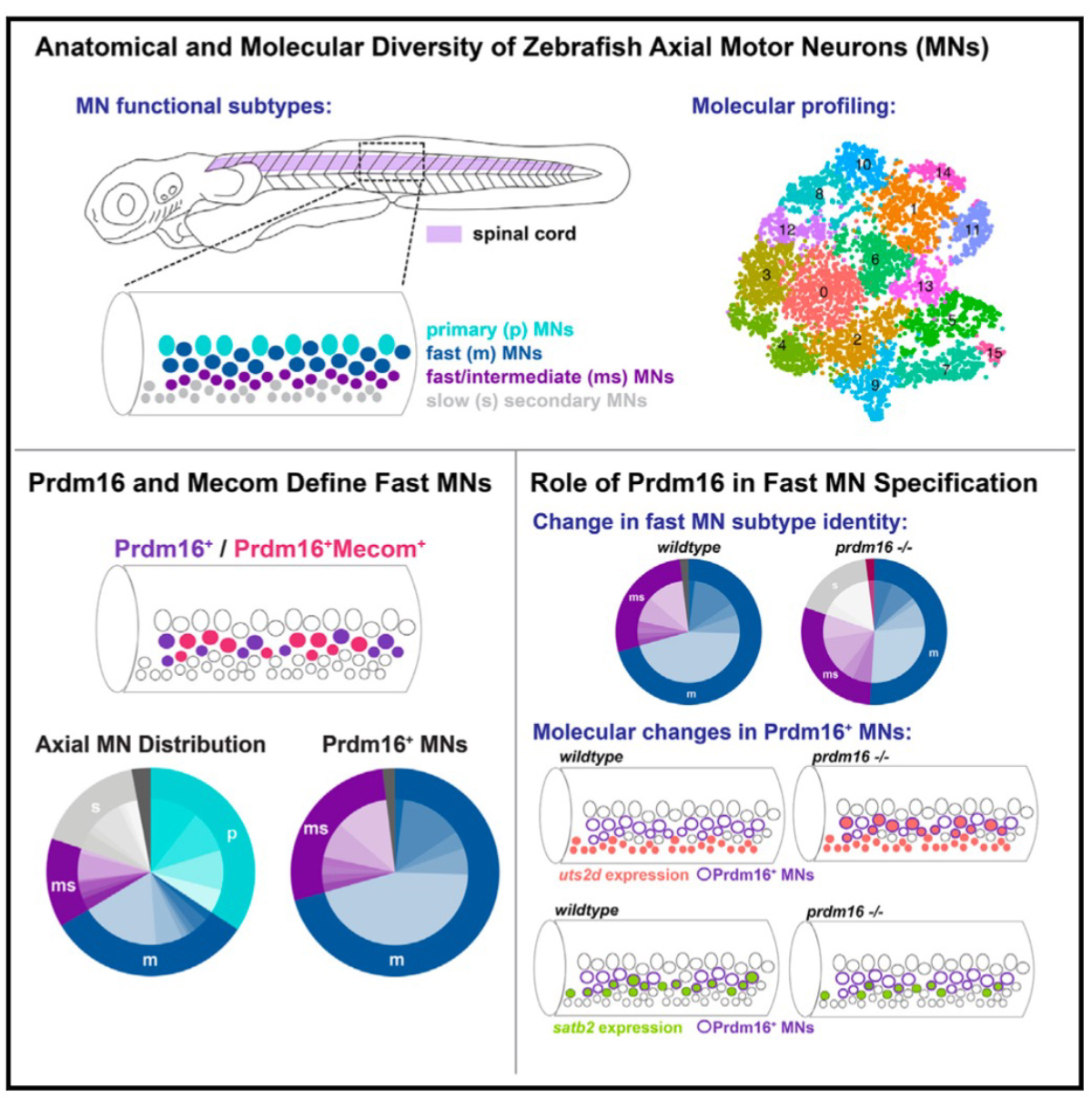
Environmental enteric dysfunction (EED) is an acquired condition of the small intestine that is associated with inflammation, poor nutrient absorption, and stunted growth in children. Malique et al. examined a cohort of children with EED and found pathophysiological changes in the cellular composition of the small bowel as well as increased serum bile acids and decreased nicotinamide adenine dinucleotide (NAD+). Work in a mouse model of EED suggested that increased dietary protein and NAD+ as well as reduction of bile acids should be further investigated as potential therapeutic avenues for this disorder. —Catherine Charneski
Abstract
Environmental enteric dysfunction (EED) is a diffuse small bowel disorder associated with poor growth, inadequate responses to oral vaccines, and nutrient malabsorption in millions of children worldwide. We identify loss of the small intestinal Paneth and goblet cells that are critical for innate immunity, reduced villous height, increased bile acids, and dysregulated nicotinamide adenine dinucleotide (NAD+) synthesis signaling as potential mechanisms underlying EED and which also correlated with diminished length-for-age z score. Isocaloric low-protein diet (LPD) consumption in mice recapitulated EED histopathology and transcriptomic changes in a microbiota-independent manner, as well as increases in serum and fecal bile acids. Children with refractory EED harbor single-nucleotide polymorphisms in key enzymes involved in NAD+ synthesis. In mice, deletion of Nampt, the gene encoding the rate-limiting enzyme in the NAD+ salvage pathway, from intestinal epithelium also reduced Paneth cell function, a deficiency that was further aggravated by LPD. Separate supplementation with NAD+ precursors or bile acid sequestrant partially restored LPD-associated Paneth cell defects and, when combined, fully restored all histopathology defects in LPD-fed mice. Therapeutic regimens that increase protein and NAD+ contents while reducing excessive bile acids may benefit children with refractory EED.