2024-02-28 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2024/02/study-proposes-streamlined-approach-to-developing-cancer-drugs.html
- https://www.nature.com/articles/s42004-024-01108-3
- https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c01502
2価のEGFRキナーゼ阻害剤でATPとアロステリック部位を連結して超加法的結合を達成 Linking ATP and allosteric sites to achieve superadditive binding with bivalent EGFR kinase inhibitors
Florian Wittlinger,Blessing C. Ogboo,Ekaterina Shevchenko,Tahereh Damghani,Calvin D. Pham,Ilse K. Schaeffner,Brandon T. Oligny,Surbhi P. Chitnis,Tyler S. Beyett,Alexander Rasch,Brian Buckley,Daniel A. Urul,Tatiana Shaurova,Earl W. May,Erik M. Schaefer,Michael J. Eck,Pamela A. Hershberger,Antti Poso,Stefan A. Laufer & David E. Heppner
Communications Chemistry Published:20 February 2024
DOI:https://doi.org/10.1038/s42004-024-01108-3
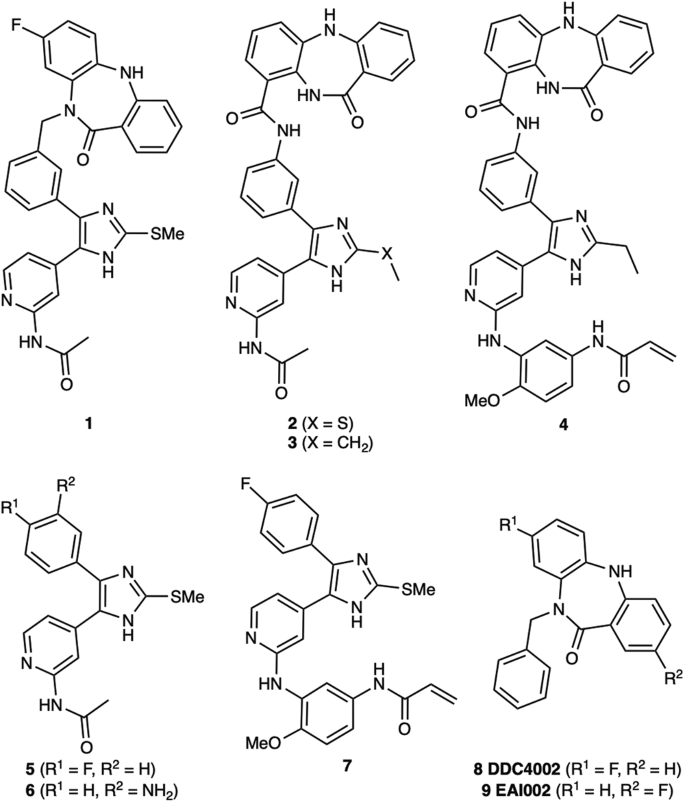
Abstract
Bivalent molecules consisting of groups connected through bridging linkers often exhibit strong target binding and unique biological effects. However, developing bivalent inhibitors with the desired activity is challenging due to the dual motif architecture of these molecules and the variability that can be introduced through differing linker structures and geometries. We report a set of alternatively linked bivalent EGFR inhibitors that simultaneously occupy the ATP substrate and allosteric pockets. Crystal structures show that initial and redesigned linkers bridging a trisubstituted imidazole ATP-site inhibitor and dibenzodiazepinone allosteric-site inhibitor proved successful in spanning these sites. The re-engineered linker yielded a compound that exhibited significantly higher potency (~60 pM) against the drug-resistant EGFR L858R/T790M and L858R/T790M/C797S, which was superadditive as compared with the parent molecules. The enhanced potency is attributed to factors stemming from the linker connection to the allosteric-site group and informs strategies to engineer linkers in bivalent agent design.
共有結合性上皮成長因子受容体阻害剤の効力と変異体選択性を決定する際の落とし穴と注意点 Pitfalls and Considerations in Determining the Potency and Mutant Selectivity of Covalent Epidermal Growth Factor Receptor Inhibitors
Kristopher W. Hoyt, Daniel A. Urul, Blessing C. Ogboo, Florian Wittlinger, Stefan A. Laufer, Erik M. Schaefer, Earl W. May, and David E. Heppner
Journal of Medicinal Chemistry Published:December 22, 2023
DOI:https://doi.org/10.1021/acs.jmedchem.3c01502
Abstract

Enzyme inhibitors that form covalent bonds with their targets are being increasingly pursued in drug development. Assessing their biochemical activity relies on time-dependent assays, which are distinct and more complex compared with methods commonly employed for reversible-binding inhibitors. To provide general guidance to the covalent inhibitor development community, we explored methods and reported kinetic values and experimental factors in determining the biochemical activity of various covalent epidermal growth factor receptor (EGFR) inhibitors. We showcase how liquid handling and assay reagents impact kinetic parameters and potency interpretations, which are critical for structure-kinetic relationships and covalent drug design. Additionally, we include benchmark kinetic values with reference inhibitors, which are imperative, as covalent EGFR inhibitor kinetic values are infrequently consistent in the literature. This overview seeks to inform best practices for developing new covalent inhibitors and highlight appropriate steps to address gaps in knowledge presently limiting assay reliability and reproducibility.


