2024-04-25 マックス・プランク研究所
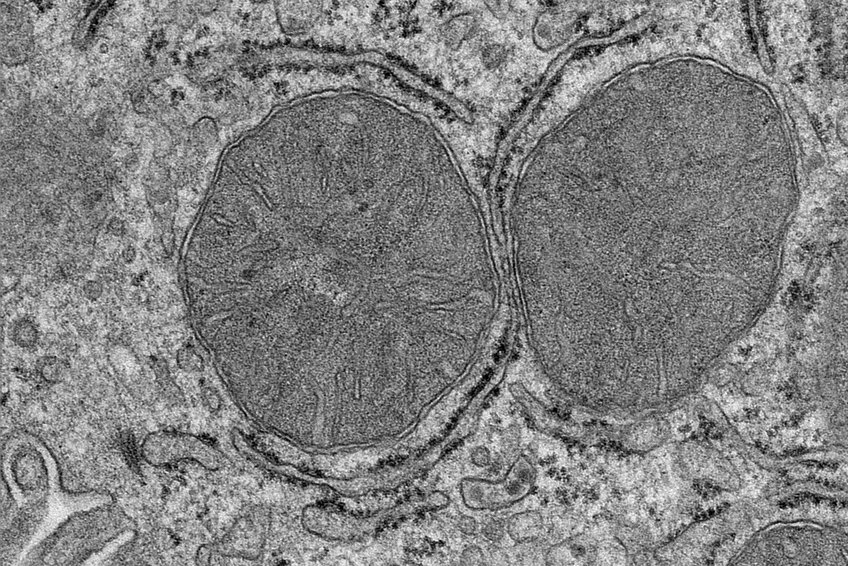
Electron micrograph of the mitochondria in liver cells. The mitochondria change their shape as soon as food is perceived. © MPI f. Metabolism Research/ S. Henschke
<関連情報>
- https://www.mpg.de/21864668/0423-neur-food-in-sight-the-liver-is-ready-153735-x?c=2249
- https://www.science.org/doi/10.1126/science.adk1005
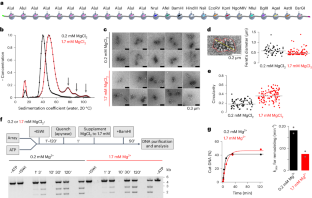
食物の知覚は肝臓におけるMFFS131のリン酸化とミトコンドリアの断片化を促進する Food perception promotes phosphorylation of MFFS131 and mitochondrial fragmentation in liver
SINIKA HENSCHKE, HENDRIK NOLTE, JUDITH MAGOLEY, TATJANA KLEELE, […], AND JENS C. BRÜNING
Science Published:25 Apr 2024
DOI:https://doi.org/10.1126/science.adk1005
Editor’s summary
Predictive responses to changes in energy status play a key role in the control of metabolic homeostasis. Metabolism-regulatory pro-opiomelanocortin (POMC)–expressing neurons in the hypothalamus are activated upon food-related cues and prime peripheral metabolism. Henschke et al. report that sensory food perception and POMC neuron activation rapidly promote mitochondrial fragmentation in the liver through activation of AKT kinase to phosphorylate the mitochondrial fission factor (MFF). A mutation that prevents phosphorylation of MFF abrogates AKT-induced mitochondrial fragmentation in response to insulin in vitro and refeeding in mice in vivo. This breakdown affects insulin’s ability to suppress hepatic glucose production. —Stella M. Hurtley
Abstract
Liver mitochondria play a central role in metabolic adaptations to changing nutritional states, yet their dynamic regulation upon anticipated changes in nutrient availability has remained unaddressed. Here, we found that sensory food perception rapidly induced mitochondrial fragmentation in the liver through protein kinase B/AKT (AKT)–dependent phosphorylation of serine 131 of the mitochondrial fission factor (MFFS131). This response was mediated by activation of hypothalamic pro-opiomelanocortin (POMC)–expressing neurons. A nonphosphorylatable MFFS131G knock-in mutation abrogated AKT-induced mitochondrial fragmentation in vitro. In vivo, MFFS131G knock-in mice displayed altered liver mitochondrial dynamics and impaired insulin-stimulated suppression of hepatic glucose production. Thus, rapid activation of a hypothalamus–liver axis can adapt mitochondrial function to anticipated changes of nutritional state in control of hepatic glucose metabolism.