2024-07-03 ロイヤルメルボルン工科大学(RMIT)
<関連情報>
- https://www.rmit.edu.au/news/all-news/2024/july/low-dose-aspirin-pregnancy
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1378610/full
- https://www.pnas.org/doi/full/10.1073/pnas.2006905117
低用量アスピリンが、A型インフルエンザウイルスに感染した妊娠マウスの大動脈内皮機能不全と胎児死亡を予防する Low dose aspirin prevents endothelial dysfunction in the aorta and foetal loss in pregnant mice infected with influenza A virus
Madison Coward-Smith,Stella Liong,Osezua Oseghale,Jonathan R. Erlich,Mark A. Miles,Felicia Liong,Kurt Brassington,Steven Bozinovski,Ross Vlahos,Robert D. Brooks,Doug A. Brooks,John J. O’Leary,Stavros Selemidis
Frontiers in Immunology Published:04 April 2024
DOI:https://doi.org/10.3389/fimmu.2024.1378610
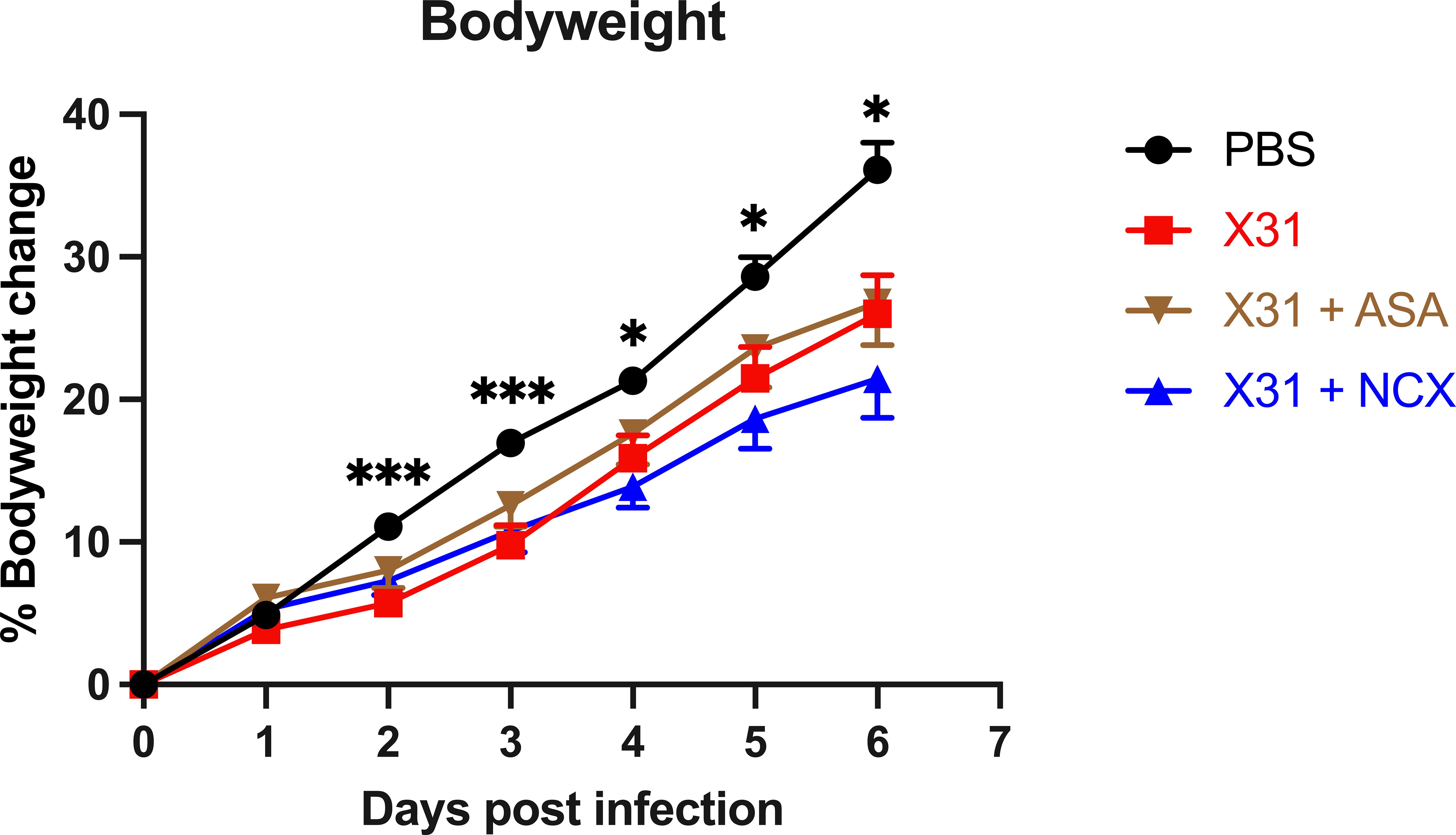
Influenza A virus (IAV) infection in pregnancy resembles a preeclamptic phenotype characterised by vascular dysfunction and foetal growth retardation. Given that low dose aspirin (ASA) is safe in pregnancy and is used to prevent preeclampsia, we investigated whether ASA or NO-conjugated aspirin, NCX4016, resolve vascular inflammation and function to improve offspring outcomes following IAV infection in pregnant mice. Pregnant mice were intranasally infected with a mouse adapted IAV strain (Hkx31; 104 plaque forming units) and received daily treatments with either 200µg/kg ASA or NCX4016 via oral gavage. Mice were then culled and the maternal lungs and aortas collected for qPCR analysis, and wire myography was performed on aortic rings to assess endothelial and vascular smooth muscle functionality. Pup and placentas were weighed and pup growth rates and survival assessed. IAV infected mice had an impaired endothelial dependent relaxation response to ACh in the aorta, which was prevented by ASA and NCX4016 treatment. ASA and NCX4016 treatment prevented IAV dissemination and inflammation of the aorta as well as improving the pup placental ratios in utero, survival and growth rates at post-natal day 5. Low dose ASA is safe to use during pregnancy for preeclampsia and this study demonstrates that ASA may prove a promising treatment for averting the significant vascular complications associated with influenza infection during pregnancy.
A型インフルエンザウイルスは、マウスにおいて自然および適応的血管炎症を介して母体および胎児の病態を引き起こす Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice
Stella Liong, Osezua Oseghale, Eunice E. To, +14, and Stavros Selemidis
Proceedings of the National Academy of Sciences Published:September 21, 2020
DOI:https://doi.org/10.1073/pnas.2006905117

Significance
Influenza infection during pregnancy is associated with increased maternal and perinatal complications. Here, we show that, during pregnancy, influenza infection leads to viral dissemination into the aorta, resulting in a peripheral “vascular storm” characterized by enhanced inflammatory mediators; the influx of Ly6C monocytes, neutrophils, and T cells; and impaired vascular function. The ensuing vascular storm induced hypoxia in the placenta and fetal brain and caused an increase in circulating cell free fetal DNA and soluble Flt1 release. We demonstrate that vascular dysfunction occurs in response to viral infection during pregnancy, which may explain the high rates of morbidity and mortality in pregnant dams, as well as the downstream perinatal complications associated with influenza infection.
Abstract
Influenza A virus (IAV) infection during pregnancy causes severe maternal and perinatal complications, despite a lack of vertical transmission of IAV across the placenta. Here, we demonstrate a significant alteration in the maternal vascular landscape that underpins the maternal and downstream fetal pathology to IAV infection in mice. In IAV infection of nonpregnant mice, the local lung inflammatory response was contained to the lungs and was self-resolving, whereas in pregnant mice, virus dissemination to major maternal blood vessels, including the aorta, resulted in a peripheral “vascular storm,” with elevated proinflammatory and antiviral mediators and the influx of Ly6Clow and Ly6Chigh monocytes, plus neutrophils and T cells. This vascular storm was associated with elevated levels of the adhesion molecules ICAM and VCAM and the pattern-recognition receptors TLR7 and TLR9 in the vascular wall, resulting in profound vascular dysfunction. The sequalae of this IAV-driven vascular storm included placental growth retardation and intrauterine growth restriction, evidence of placental and fetal brain hypoxia, and increased circulating cell free fetal DNA and soluble Flt1. In contrast, IAV infection in nonpregnant mice caused no obvious alterations in endothelial function or vascular inflammation. Therefore, IAV infection during pregnancy drives a significant systemic vascular alteration in pregnant dams, which likely suppresses critical blood flow to the placenta and fetus. This study in mice provides a fundamental mechanistic insight and a paradigm into how an immune response to a respiratory virus, such as IAV, is likely to specifically drive maternal and fetal pathologies during pregnancy.