2024-11-12 ゲーテ大学
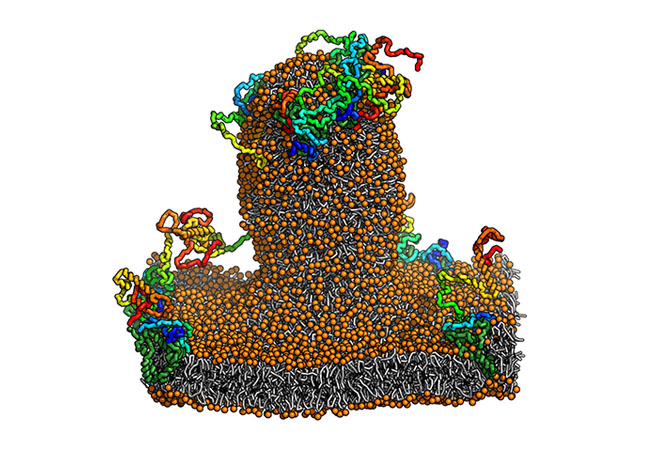
Detail of a membrane bubble. Image: Bhaskara Group, Goethe University Frankfurt
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/bulges-calculated-in-the-supercomputer-how-cells-digest-their-internal-canal-system/
- https://www.pnas.org/doi/10.1073/pnas.2408071121
選択的小胞体貪食を増強する膜リモデリングを増幅する本質的に無秩序な領域 Intrinsically disordered region amplifies membrane remodeling to augment selective ER-phagy
Sergio Alejandro Poveda-Cuevas, Kateryna Lohachova, Borna Markusic, +2, and Ramachandra M. Bhaskara
Proceedings of the National Academy of Sciences Published:October 25, 2024
DOI:https://doi.org/10.1073/pnas.2408071121
Significance
The extent of intrinsic disorder in membrane remodeling remains unclear. Here, we focus on the C-terminal disordered region of FAM134B, a protein involved in the endoplasmic reticulum (ER) recycling process. Through advanced computer modeling and extensive simulations, we show how membrane-anchored disordered protein segments exhibit different ensemble properties. This context-dependent behavior is sequence-encoded and shared among other proteins involved in ER-phagy. Membrane anchoring alone enables disordered regions to sense and influence local membrane shape, and when combined with other membrane-shaping elements, they accelerate large-scale membrane remodeling. These insights deepen our understanding of disordered segments and how they influence membrane shapes.
Abstract
Intrinsically disordered regions (IDRs) play a pivotal role in organellar remodeling. They transduce signals across membranes, scaffold signaling complexes, and mediate vesicular traffic. Their functions are regulated by constraining conformational ensembles through specific intra- and intermolecular interactions, physical tethering, and posttranslational modifications. The endoplasmic reticulum (ER)-phagy receptor FAM134B/RETREG1, known for its reticulon homology domain (RHD), includes a substantial C-terminal IDR housing the LC3 interacting motif. Beyond engaging the autophagic machinery, the function of the FAM134B-IDR is unclear. Here, we investigate the characteristics of the FAM134B-IDR by extensive modeling and molecular dynamics simulations. We present detailed structural models for the IDR, mapping its conformational landscape in solution and membrane-anchored configurations. Our analysis reveals that depending on the membrane anchor, the IDRs collapse onto the membrane and induce positive membrane curvature to varying degrees. The charge patterns underlying this Janus-like behavior are conserved across other ER-phagy receptors. We found that IDRs alone are sufficient to sense curvature. When combined with RHDs, they intensify membrane remodeling and drive efficient protein clustering, leading to faster budding, thereby amplifying RHD remodeling functions. Our simulations provide a perspective on IDRs of FAM134B, their Janus-like membrane interactions, and the resulting modulatory functions during large-scale ER remodeling.