2024-12-05 ワシントン大学セントルイス校
<関連情報>
- https://news.wsu.edu/press-release/2024/12/05/potential-epigenetic-biomarker-found-for-preeclampsia-in-pregnancy/
- https://academic.oup.com/eep/article/10/1/dvae022/7877253
- https://www.nature.com/articles/s41598-022-07262-9
子癇関連早産のエピジェネティックバイオマーカーと予防医学の可能性 Epigenetic biomarker for preeclampsia-associated preterm birth and potential preventative medicine
Eric E Nilsson, Paul Winchester, Cathy Proctor, Daniel Beck, Michael K Skinner
Environmental Epigenetics Published:06 November 2024
DOI:https://doi.org/10.1093/eep/dvae022
Abstract
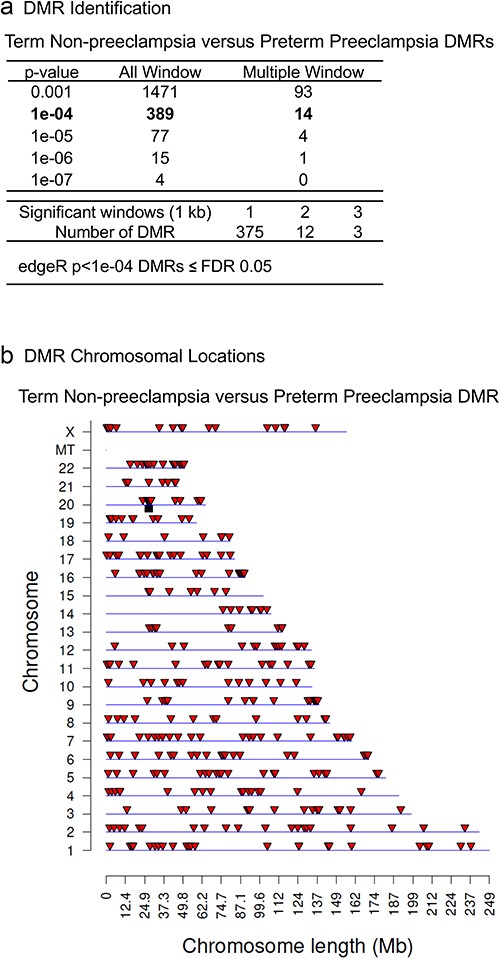
Preterm birth (PTB) has dramatically increased within the population (i.e. >10%) and preeclampsia is a significant sub-category of PTB. Currently, there are no practical clinical parameters or biomarkers which predict preeclampsia induced PTB. The current study investigates the potential use of epigenetic (DNA methylation) alterations as a maternal preeclampsia biomarker. Non-preeclampsia term births were compared to preeclampsia PTBs to identify DNA methylation differences (i.e. potential epigenetic biomarker). Maternal buccal cell cheek swabs were used as a marker cell for systemic epigenetic alterations in the individuals, which are primarily due to environmentally induced early life or previous generations impacts, and minimally impacted or associated with the disease etiology or gestation variables. A total of 389 differential DNA methylation regions (DMRs) were identified and associated with the presence of preeclampsia. The DMRs were genome-wide and were predominantly low CpG density (<2 CpG/100 bp). In comparison with a previous PTB buccal cell epigenetic biomarker there was a 15% (60 DMR) overlap, indicating that the majority of the DMRs are unique for preeclampsia. Few previously identified preeclampsia genes have been identified, however, the DMRs had gene associations in the P13 K-Akt signaling pathway and metabolic gene family, such as phospholipid signaling pathway. Preliminary validation of the DMR use as a potential maternal biomarker used a cross-validation analysis on the samples and provided 78% accuracy. Although prospective expanded clinical trials in first trimester pregnancies and clinical comparisons are required, the current study provides the potential proof of concept a preeclampsia epigenetic biomarker may exist. The availability of a preeclampsia PTB maternal susceptibility biomarker may facilitate clinical management and allow preventative medicine approaches to identify and treat the preeclampsia condition prior to its occurrence.
早産の頬側細胞のエピジェネティックバイオマーカーが予防医療を促進する Preterm birth buccal cell epigenetic biomarkers to facilitate preventative medicine
Paul Winchester,Eric Nilsson,Daniel Beck & Michael K. Skinner
Scientific Reports Published:01 March 2022
DOI:https://doi.org/10.1038/s41598-022-07262-9
Abstract
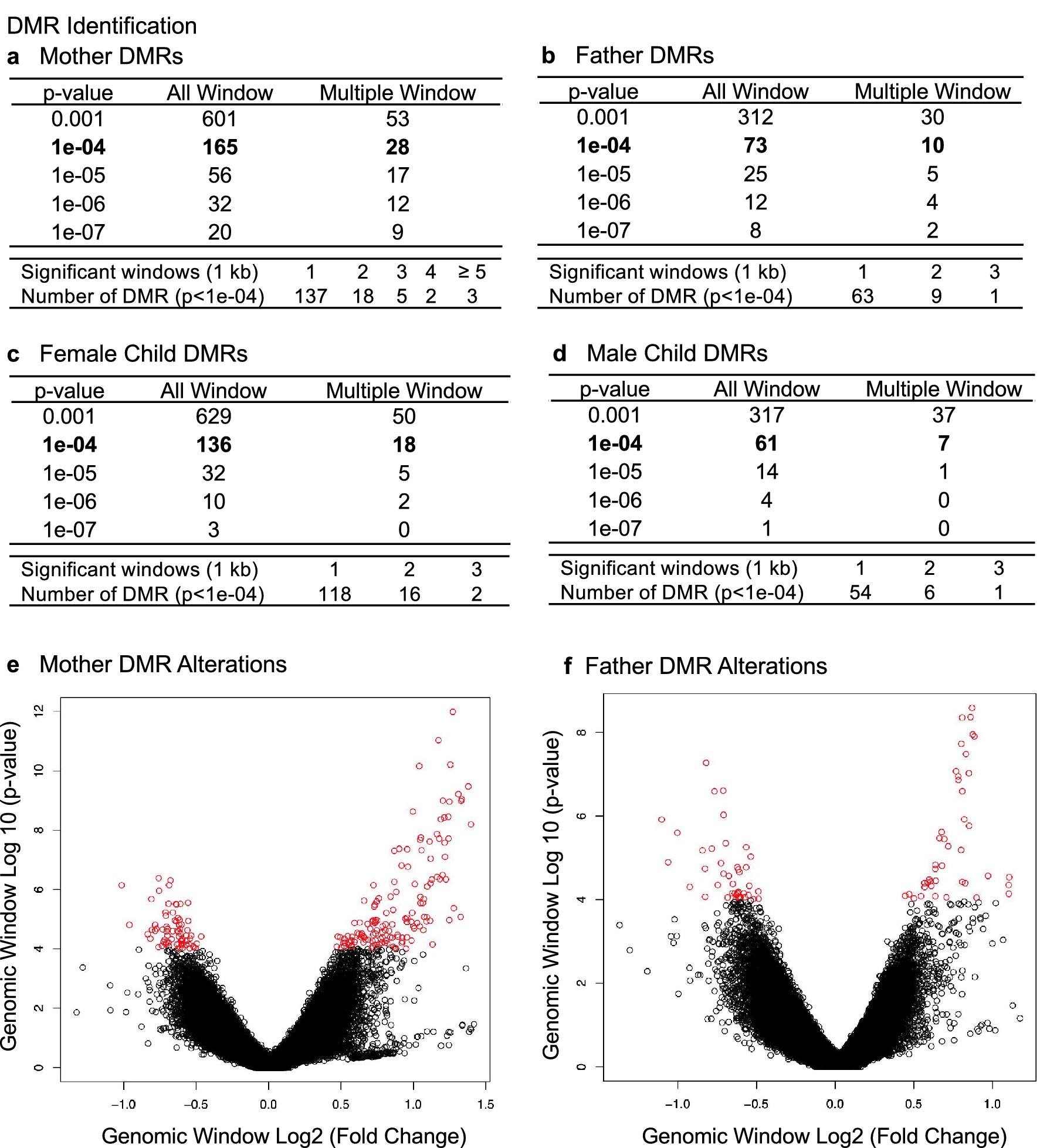
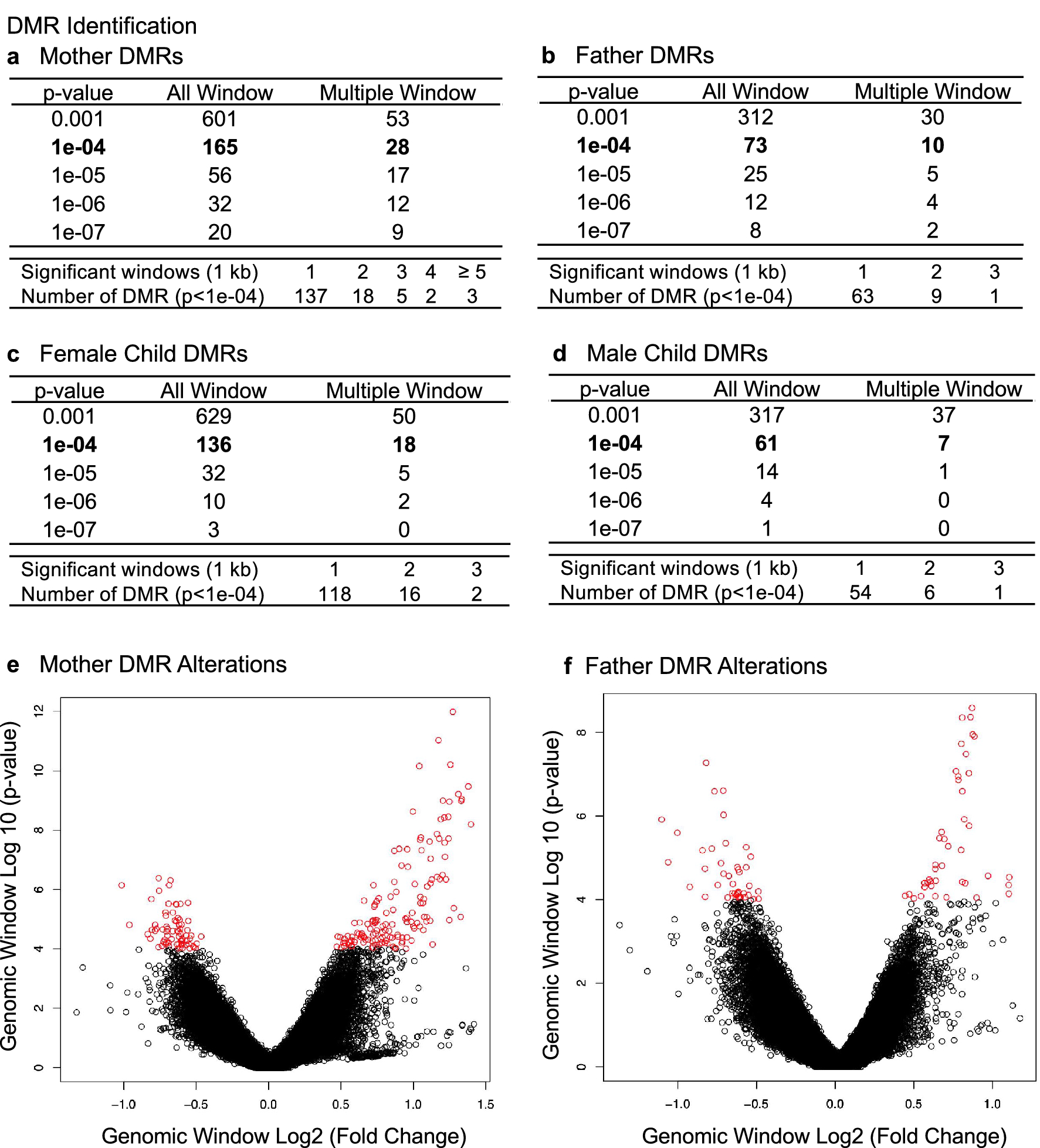
Preterm birth is the major cause of newborn and infant mortality affecting nearly one in every ten live births. The current study was designed to develop an epigenetic biomarker for susceptibility of preterm birth using buccal cells from the mother, father, and child (triads). An epigenome-wide association study (EWAS) was used to identify differential DNA methylation regions (DMRs) using a comparison of control term birth versus preterm birth triads. Epigenetic DMR associations with preterm birth were identified for both the mother and father that were distinct and suggest potential epigenetic contributions from both parents. The mother (165 DMRs) and female child (136 DMRs) at p < 1e-04 had the highest number of DMRs and were highly similar suggesting potential epigenetic inheritance of the epimutations. The male child had negligible DMR associations. The DMR associated genes for each group involve previously identified preterm birth associated genes. Observations identify a potential paternal germline contribution for preterm birth and identify the potential epigenetic inheritance of preterm birth susceptibility for the female child later in life. Although expanded clinical trials and preconception trials are required to optimize the potential epigenetic biomarkers, such epigenetic biomarkers may allow preventative medicine strategies to reduce the incidence of preterm birth.