2024-12-18 ニューヨーク大学 (NYU)
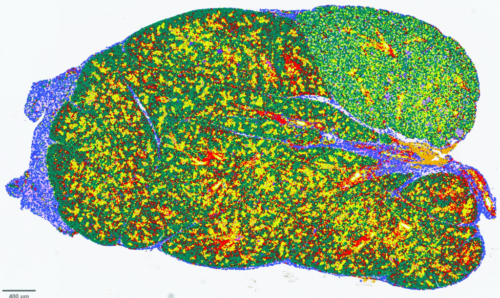
Studying salivary glands in a mouse model of Sjögren’s disease, NYU researchers used machine learning to detect different cell types, each represented by different colors. Reprinted with permission from Wang et al., Sci. Transl. Med. 16, eado4856 (2024) 18 December 2024
<関連情報>
- https://www.nyu.edu/about/news-publications/news/2024/december/-sjogrens-disease.html
- https://www.science.org/doi/10.1126/scitranslmed.ado4856
- https://journals.physiology.org/doi/full/10.1093/function/zqae047
IFN-γ産生TH1細胞と機能不全制御性T細胞はシェーグレン病の病態に寄与する IFN-γ–producing TH1 cells and dysfunctional regulatory T cells contribute to the pathogenesis of Sjögren’s disease
Yin-Hu Wang, Wenyi Li, Maxwell McDermott, Ga-Yeon Son, […], and Stefan Feske
Science Translational Medicine Published:18 Dec 2024
DOI:https://doi.org/10.1126/scitranslmed.ado4856
Editor’s summary
The drivers of Sjögren’s disease (SjD), an autoimmune disease primarily characterized by immune-mediated dysfunction and destruction of salivary and lacrimal glands, are not well understood. Here, Wang et al. developed and characterized a genetic mouse model that closely mirrors human SjD. The mice, which have dysfunctional regulatory T cells, develop inflammation in their salivary and lacrimal glands similar to that in humans, and the authors found that this inflammation was driven by interferon-γ–producing CD4+ T cells. Importantly, baricitinib treatment alleviated the pathogenic effects of CD4+ T cells in this SjD-like mouse model, hinting at a potential therapeutic approach for patients with SjD. —Courtney Malo
Abstract
Sjögren’s disease (SjD) is an autoimmune disorder characterized by progressive salivary and lacrimal gland dysfunction, inflammation, and destruction, as well as extraglandular manifestations. SjD is associated with autoreactive B and T cells, but its pathophysiology remains incompletely understood. Abnormalities in regulatory T (Treg) cells occur in several autoimmune diseases, but their role in SjD is ambiguous. We had previously shown that the function and development of Treg cells depend on store-operated Ca2+ entry (SOCE), which is mediated by ORAI1 Ca2+ channels and stromal interaction protein 1 (STIM1) and STIM2. Here, we show that mice with a Foxp3+ Treg cell–specific deletion of Stim1 and Stim2 develop a phenotype that fulfills all classification criteria of human SjD. Mutant mice have salivary and lacrimal gland inflammation characterized by strong lymphocyte infiltration and transcriptional signatures dominated by T helper 1 (TH1) and interferon (IFN) signaling. CD4+ T cells from mutant mice are sufficient to induce SjD-like disease in an IFN-γ–dependent manner. Inhibition of IFN signaling with the JAK1/2 inhibitor baricitinib alleviated CD4+ T cell–induced SjD in mice. These findings are consistent with the transcriptional profiles of CD4+ T cells from patients with SjD, which indicate enhanced TH1 but reduced memory Treg cell function. Together, our study provides evidence for a critical role of dysfunctional Treg cells and IFN-γ–producing TH1 cells in the pathogenesis of SjD.
唾液腺におけるSTIM1およびSTIM2の欠損はANO1の機能を破壊するが、シェーグレン病を誘発しない Loss of STIM1 and STIM2 in salivary glands disrupts ANO1 function but does not induce Sjogren’s disease
Ga-Yeon Son, Anna Zou, Amanda Wahl, Kai Ting Huang, Saruul Zorgit, Manikandan Vinu, Fang Zhou, Larry Wagner, Youssef Idaghdour, David I Yule …
Function Published:30 October 2024
DOI:https://doi.org/10.1093/function/zqae047

Graphical Abstract
Abstract
Ca2+ signaling via the store operated Ca2+ entry (SOCE) mediated by STIM1 and STIM2 proteins and the ORAI1 Ca2+ channel is important in saliva fluid secretion and has been associated with Sjogren’s disease (SjD). However, there are no studies addressing STIM1/2 dysfunction in salivary glands or SjD in animal models. We report that mice lacking Stim1 and Stim2 (Stim1/2K14Cre(+)) in salivary glands exhibited reduced Ca2+ levels and hyposalivate. SOCE was functionally required for the activation of the Ca2+ activated Cl– channel ANO1. Ageing Stim1/2K14Cre(+) mice showed no evidence of lymphocytic infiltration or increased levels of autoantibodies characteristic of SjD, possibly associated with a downregulation of toll-like receptor 8 (Tlr8) expression. Salivary gland biopsies of SjD patients showed increased expression of STIM1 and TLR7/8. Our study shows that SOCE activates ANO1 function and fluid secretion in salivary glands and highlights a potential link between SOCE and TLR signaling in SjD.

