2025-01-08 バース大学
<関連情報>
- https://www.bath.ac.uk/announcements/research-helps-predict-which-melanoma-patients-are-most-likely-to-respond-to-immunotherapy/
- https://ascopubs.org/doi/full/10.1200/OA-24-00049
ステージII黒色腫におけるネオアジュバント腫瘍溶解性ウイルスに対する反応性の機能的バイオマーカーに向けて:免疫フェルスター共鳴エネルギー移動と動的腫瘍免疫微小環境 Toward Functional Biomarkers of Response to Neoadjuvant Oncolytic Virus in Stage II Melanoma: Immune-Förster Resonance Energy Transfer and the Dynamic Tumor Immune Microenvironment
Amanda R. Kirane, MD, PhD , David Lee, BS, Michael Lowe, MD , M. Usman Ahmad, MD, Saurabh Sharma, PhD, Mamatha Serasanambati, PhD, Christopher J. Applebee, MSc, … and Emanual Maverakis, MD
JCO Oncology Advances Published:January 02, 2025
DOI:https://doi.org/10.1200/OA-24-00049

Abstract
Purpose
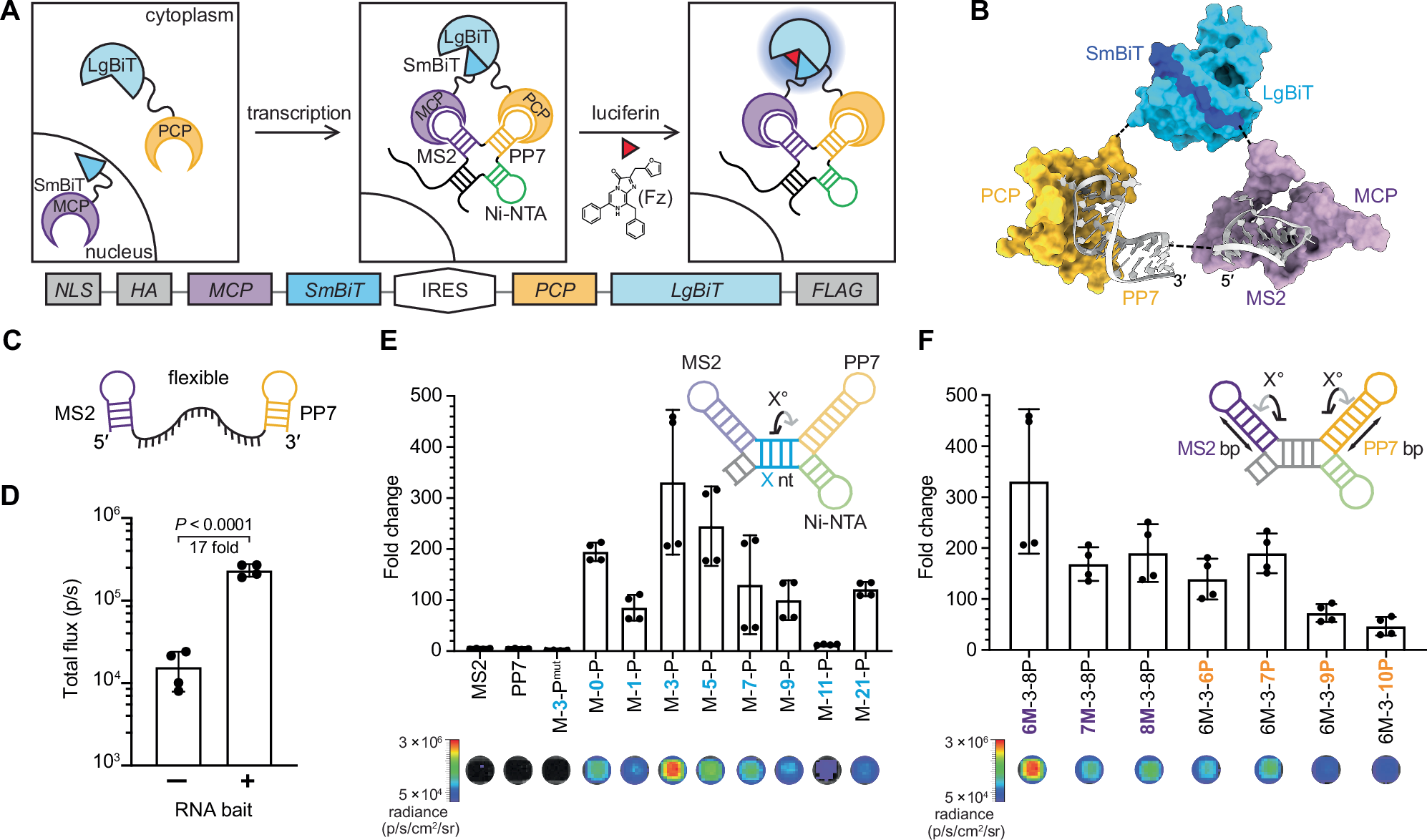
Despite the effectiveness of immunotherapy in advanced melanoma, the lack of clinically reliable biomarkers hinders precision medicine approaches. This study investigates the potential of immune time-resolved Förster resonance energy transfer (iFRET) as a novel tool for understanding immunotherapy response in melanoma.
Methods
Using tissue from a pilot phase II study of neoadjuvant talimogene laherparepvec (TVEC) before standard surgery, we explore iFRET’s ability to assess PD-L1:PD-1 interactions in the tumor immune microenvironment (TiME) pre- and post-therapy.
Results
Responsive tumors demonstrated significant increases in iFRET efficiency, diverging from nonresponsive tumors that either decreased in checkpoint engagement or failed to demonstrate immune stimulation with therapy. Changes in PD-L1:PD-1 iFRET efficiency did not correlate with changes in PD-L1 expression. Instead, traditional biomarkers of PD-L1 expression and T-cell phenotyping did not reflect response trends and demonstrated significant heterogeneity inter- and intratumorally. Importantly, tumor-associated macrophage phenotype correlated significantly with TVEC response and the significantly high PD-L1:PD-1 interaction observed in the tumor beds of complete responders.
Conclusion
These findings underscore both the role of innate immune profiles in immunotherapy outcomes and the potential of iFRET to serve as a critical companion diagnostic in the classification of immune profiles. This research reveals crucial insights into factors affecting checkpoint function in tumors and emphasizes the need for further investigation into cell-specific interactions within the TiME. By expanding our understanding of distinct patient profiles for tailored therapeutic strategies, we underscore the importance of assessing this type of functional biomarker data in ongoing neoadjuvant trial designs to advance the goal of precision immunotherapy in patients with melanoma.