2025-03-24 横浜市立大学
<関連情報>
- https://www.yokohama-cu.ac.jp/res-portal/news/2024/20250324nishimura.html
- https://www.yokohama-cu.ac.jp/res-portal/news/2024/gjok7g0000000l3r-att/20250324nishimura.pdf
- https://academic.oup.com/nar/article/53/6/gkaf154/8089752
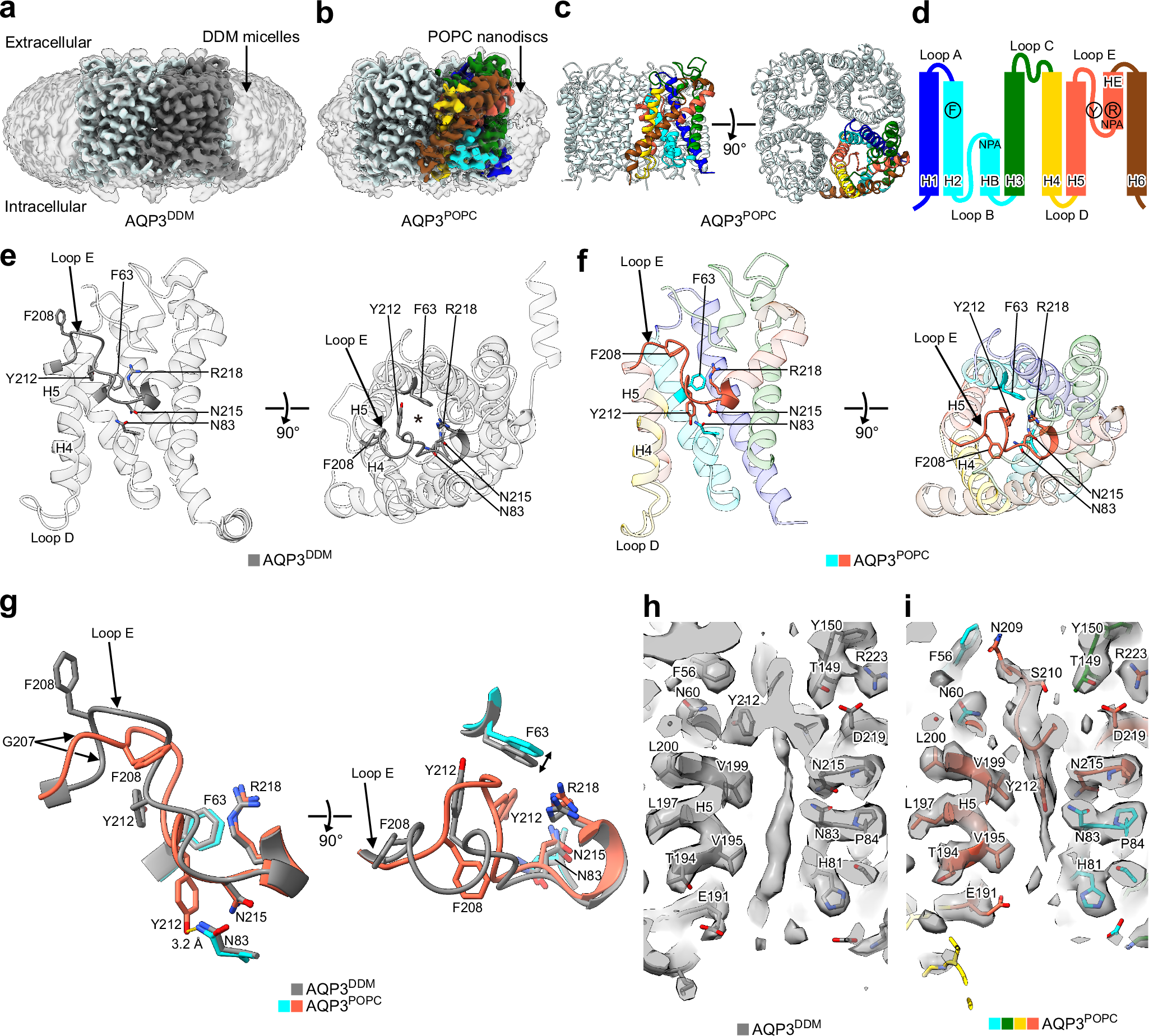
統合的構造解析によって明らかになった相分離ヘテロクロマチンタンパク質1αの動的構造単位 A dynamic structural unit of phase-separated heterochromatin protein 1α as revealed by integrative structural analyses
Ayako Furukawa, Kento Yonezawa, Tatsuki Negami, Yuriko Yoshimura, Aki Hayashi, Jun-ichi Nakayama, Naruhiko Adachi, Toshiya Senda, Kentaro Shimizu, Tohru Terada, …
Nucleic Acids Research Published:24 March 2025
DOI:https://doi.org/10.1093/nar/gkaf154
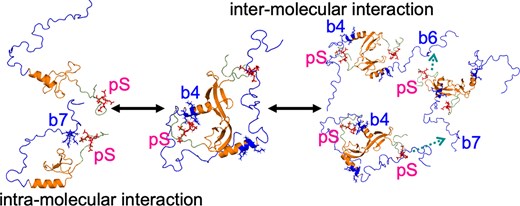
Graphical Abstract
Abstract
The heterochromatin protein HP1α consists of an N-terminal disordered tail (N-tail), chromodomain (CD), hinge region (HR), and C-terminal chromo shadow domain (CSD). While CD binds to the lysine9-trimethylated histone H3 (H3K9me3) tail in nucleosomes, CSD forms a dimer bridging two nucleosomes with H3K9me3. Phosphorylation of serine residues in the N-tail enhances both H3K9me3 binding and liquid–liquid phase separation (LLPS) by HP1α. We have used integrative structural methods, including nuclear magnetic resonance, small-angle X-ray scattering (SAXS), and multi-angle-light scattering combined with size-exclusion chromatography, and coarse-grained molecular dynamics simulation with SAXS, to probe the HP1α dimer and its CSD deletion monomer. We show that dynamic intra- and intermolecular interactions between the N-tails and basic segments in CD and HR depend on N-tail phosphorylation. While the phosphorylated HP1α dimer undergoes LLPS via the formation of aggregated multimers, the N-tail phosphorylated mutant without CSD still undergoes LLPS, but its structural unit is a dynamic intermolecular dimer formed via the phosphorylated N-tail and a basic segment at the CD end. Furthermore, we reveal that mutation of this basic segment in HP1α affects the size of heterochromatin foci in cultured mammalian cells, suggesting that this interaction plays an important role in heterochromatin formation in vivo.