2025-04-07 マサチューセッツ工科大学(MIT)
<関連情報>
- https://news.mit.edu/2025/molecules-fighting-infection-act-in-brain-inducing-anxiety-or-sociability-0407
- https://www.cell.com/cell/abstract/S0092-8674(25)00278-8
- https://www.cell.com/cell/abstract/S0092-8674(25)00279-X
- https://www.nature.com/articles/s41586-019-1843-6
炎症性サイトカインと抗炎症性サイトカインが不安を制御する扁桃体回路を双方向に調節する Inflammatory and anti-inflammatory cytokines bidirectionally modulate amygdala circuits regulating anxiety
Byeongjun Lee∙ Jeong-Tae Kwon∙ Yire Jeong∙ … ∙ Kwanghun Chung∙ Jun R. Huh∙ Gloria B. Choi
Cell Published:April 7, 2025
DOI:https://doi.org/10.1016/j.cell.2025.03.005
Graphical abstract

Highlights
- Disrupting peripheral IL-17RA boosts IL-17A/IL-17C and reduces IL-10 in circulation
- IL-17RA, found in glutamatergic BLAa neurons, coexpresses IL-17RE and IL-10RA
- IL-17A/IL-17C raises BLAa neuron excitability via IL-17RA, inducing anxiety phenotypes
- IL-10/IL-10RA signaling reduces BLAa neuron excitability and is anxiolytic
Summary
Patients with autoimmune or infectious diseases can develop persistent mood alterations after inflammatory episodes. Peripheral immune molecules, like cytokines, can influence behavioral and internal states, yet their impact on the function of specific neural circuits in the brain remains unclear. Here, we show that cytokines act as neuromodulators to regulate anxiety by engaging receptor-expressing neurons in the basolateral amygdala (BLA). Heightened interleukin-17A (IL-17A) and IL-17C levels, paradoxically induced from treatment with anti-IL-17 receptor A (IL-17RA) antibodies, promote anxiogenic behaviors by increasing the excitability of IL-17RA/RE-expressing BLA neurons. Conversely, the anti-inflammatory IL-10, acting on the same population of BLA neurons via its receptor, exerts opposite effects on neuronal excitability and behavior. These findings reveal that inflammatory and anti-inflammatory cytokines bidirectionally modulate anxiety by engaging their respective receptors in the same BLA population. Our results highlight the role of cytokine signaling in shaping internal states through direct modulation of specific neural substrates.
IL-17Eと受容体IL-17RBが神経調節に関与していることが、免疫受容体の脳全体にわたるマッピングから明らかになった Brain-wide mapping of immune receptors uncovers a neuromodulatory role of IL-17E and the receptor IL-17RB
Yunjin Lee∙ Tomoe Ishikawa∙ Hyeseung Lee∙ … ∙ Myriam Heiman∙ Gloria B. Choi∙ Jun R. Huh
Cell Published:April 7, 2025
DOI:https://doi.org/10.1016/j.cell.2025.03.006
Graphical abstract

Highlights
•IL-17 receptor subunits exhibit distinct neuronal expression patterns in the brain
•IL-17RB and its interaction with IL-17E in the S1DZ promote social behaviors
•IL-17E functions as a neuromodulator in cortical neurons
Summary
Cytokines interact with their receptor complexes to orchestrate diverse processes—from immune responses to behavioral modulation. Interleukin-17A (IL-17A) mediates protective immune responses by binding to IL-17 receptor A (IL-17RA) and IL-17RC subunits. IL-17A also modulates social interaction, yet the role of cytokine receptors in this process and their expression in the brain remains poorly characterized. Here, we mapped the brain-region-specific expression of all major IL-17R subunits and found that in addition to IL-17RA, IL-17RB—but not IL-17RC—plays a role in social behaviors through its expression in the cortex. We further showed that IL-17E, expressed in cortical neurons, enhances social interaction by acting on IL-17RA- and IL-17RB-expressing neurons. These findings highlight an IL-17 circuit within the cortex that modulates social behaviors. Thus, characterizing spatially restricted cytokine receptor expression can be leveraged to elucidate how cytokines function as critical messengers mediating neuroimmune interactions to shape animal behaviors.
IL-17aは神経発達障害モデルマウスにおいて社交性を促進する IL-17a promotes sociability in mouse models of neurodevelopmental disorders
Michael Douglas Reed,Yeong Shin Yim,Ralf D. Wimmer,Hyunju Kim,Changhyeon Ryu,Gwyneth Margaret Welch,Matias Andina,Hunter Oren King,Ari Waisman,Michael M. Halassa,Jun R. Huh & Gloria B. Choi
Nature Published:18 December 2019
DOI:https://doi.org/10.1038/s41586-019-1843-6
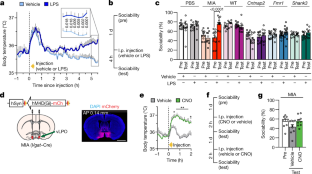
Abstract
A subset of children with autism spectrum disorder appear to show an improvement in their behavioural symptoms during the course of a fever, a sign of systemic inflammation1,2. Here we elucidate the molecular and neural mechanisms that underlie the beneficial effects of inflammation on social behaviour deficits in mice. We compared an environmental model of neurodevelopmental disorders in which mice were exposed to maternal immune activation (MIA) during embryogenesis3,4 with mouse models that are genetically deficient for contactin-associated protein-like 2 (Cntnap2)5, fragile X mental retardation-1 (Fmr1)6 or Sh3 and multiple ankyrin repeat domains 3 (Shank3)7. We establish that the social behaviour deficits in offspring exposed to MIA can be temporarily rescued by the inflammatory response elicited by the administration of lipopolysaccharide (LPS). This behavioural rescue was accompanied by a reduction in neuronal activity in the primary somatosensory cortex dysgranular zone (S1DZ), the hyperactivity of which was previously implicated in the manifestation of behavioural phenotypes associated with offspring exposed to MIA8. By contrast, we did not observe an LPS-induced rescue of social deficits in the monogenic models. We demonstrate that the differences in responsiveness to the LPS treatment between the MIA and the monogenic models emerge from differences in the levels of cytokine production. LPS treatment in monogenic mutant mice did not induce amounts of interleukin-17a (IL-17a) comparable to those induced in MIA offspring; bypassing this difference by directly delivering IL-17a into S1DZ was sufficient to promote sociability in monogenic mutant mice as well as in MIA offspring. Conversely, abrogating the expression of IL-17 receptor subunit a (IL-17Ra) in the neurons of the S1DZ eliminated the ability of LPS to reverse the sociability phenotypes in MIA offspring. Our data support a neuroimmune mechanism that underlies neurodevelopmental disorders in which the production of IL-17a during inflammation can ameliorate the expression of social behaviour deficits by directly affecting neuronal activity in the central nervous system.