2025-04-23 アメリカ国立衛生研究所 (NIH)
<関連情報>
- https://www.nih.gov/news-events/news-releases/nih-study-reveals-how-inflammation-makes-touch-painful
- https://www.nature.com/articles/s41586-025-08875-6
- https://www.science.org/doi/10.1126/scitranslmed.aat9892
- https://www.science.org/doi/10.1126/scitranslmed.aat9897
熱感覚と炎症性疼痛の分散型コード化ロジック A distributed coding logic for thermosensation and inflammatory pain
Nima Ghitani,Lars J. von Buchholtz,Donald Iain MacDonald,Melanie Falgairolle,Minh Q. Nguyen,Julia A. Licholai,Nicholas J. P. Ryba & Alexander T. Chesler
Nature Published:23 April 2025
DOI:https://doi.org/10.1038/s41586-025-08875-6
Abstract
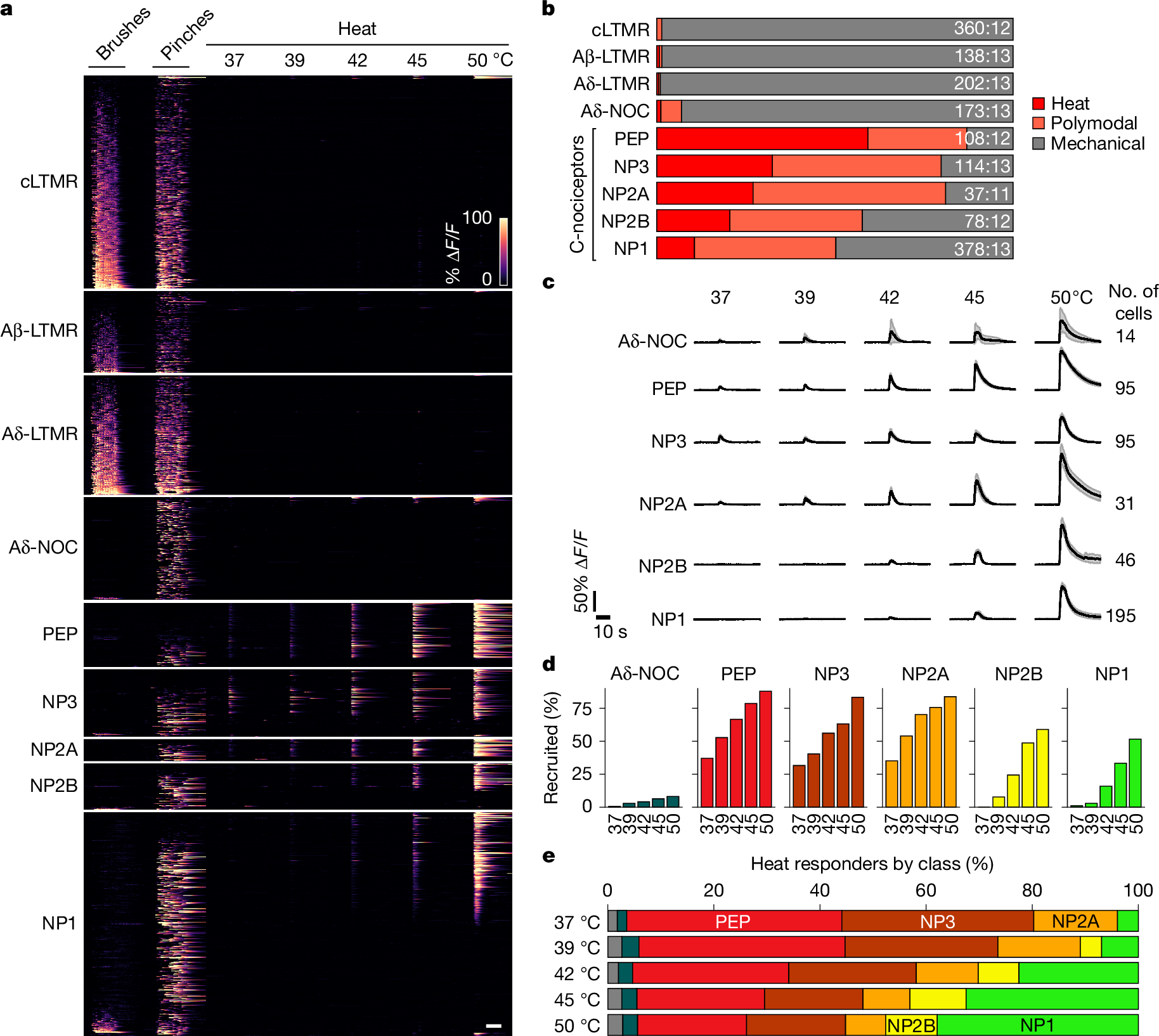
Somatosensory neurons encode detailed information about touch and temperature and are the peripheral drivers of pain1,2. Here by combining functional imaging with multiplexed in situ hybridization3, we determined how heat and mechanical stimuli are encoded across neuronal classes and how inflammation transforms this representation to induce heat hypersensitivity, mechanical allodynia and continuing pain. Our data revealed that trigeminal neurons innervating the cheek exhibited complete segregation of responses to gentle touch and heat. By contrast, heat and noxious mechanical stimuli broadly activated nociceptor classes, including cell types proposed to trigger select percepts and behaviours4,5,6. Injection of the inflammatory mediator prostaglandin E2 caused long-lasting activity and thermal sensitization in select classes of nociceptors, providing a cellular basis for continuing inflammatory pain and heat hypersensitivity. We showed that the capsaicin receptor TRPV1 (ref. 7) has a central role in heat sensitization but not in spontaneous nociceptor activity. Unexpectedly, the responses to mechanical stimuli were minimally affected by inflammation, suggesting that tactile allodynia results from the continuing firing of nociceptors coincident with touch. Indeed, we have demonstrated that nociceptor activity is both necessary and sufficient for inflammatory tactile allodynia. Together, these findings refine models of sensory coding and discrimination at the cellular and molecular levels, demonstrate that touch and temperature are broadly but differentially encoded across transcriptomically distinct populations of sensory cells and provide insight into how cellular-level responses are reshaped by inflammation to trigger diverse aspects of pain.
PIEZO2がマウスとヒトの傷害誘発触覚痛を媒介する PIEZO2 mediates injury-induced tactile pain in mice and humans
Marcin Szczot, Jaquette Liljencrantz, Nima Ghitani, Arnab Barik, […], and Alexander T. Chesler
Science Translational Medicine Published:10 Oct 2018
DOI:https://doi.org/10.1126/scitranslmed.aat9892
Understanding pain sensitization
Inflammation or nerve injury can alter tactile sensation, making a gentle touch perceived as painful. The molecular mechanisms mediating this alteration in sensation, called mechanical allodynia, are not completely understood. Murthy et al. and Szczot et al. found that PIEZO2 ion channels expressed in sensory neurons are required for the development of mechanical allodynia in mice and humans. The authors independently reached this conclusion using different animal models. Szczot et al. extended the discovery to humans, showing that PIEZO2 loss of function resulted in failure to develop mechanical allodynia. These two studies suggest that local inhibition of PIEZO2 ion channels might be effective for treating mechanical allodynia.
Abstract
Tissue injury and inflammation markedly alter touch perception, making normally innocuous sensations become intensely painful. Although this sensory distortion, known as tactile allodynia, is one of the most common types of pain, the mechanism by which gentle mechanical stimulation becomes unpleasant remains enigmatic. The stretch-gated ion channel PIEZO2 has been shown to mediate light touch, vibration detection, and proprioception. However, the role of this ion channel in nociception and pain has not been resolved. Here, we examined the importance of Piezo2 in the cellular representation of mechanosensation using in vivo imaging in mice. Piezo2-knockout neurons were completely insensitive to gentle dynamic touch but still responded robustly to noxious pinch. During inflammation and after injury, Piezo2 remained essential for detection of gentle mechanical stimuli. We hypothesized that loss of PIEZO2 might eliminate tactile allodynia in humans. Our results show that individuals with loss-of-function mutations in PIEZO2 completely failed to develop sensitization and painful reactions to touch after skin inflammation. These findings provide insight into the basis for tactile allodynia, identify the PIEZO2 mechanoreceptor as an essential mediator of touch under inflammatory conditions, and suggest that this ion channel might be targeted for treating tactile allodynia.
機械感受性イオンチャネルPiezo2がマウスの機械的痛みに対する感受性を媒介する The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice
Swetha E. Murthy, Meaghan C. Loud, Ihab Daou, Kara L. Marshall, […] , and Ardem Patapoutian
Science Translational Medicine Published:10 Oct 2018
DOI:https://doi.org/10.1126/scitranslmed.aat9897
Understanding pain sensitization
Inflammation or nerve injury can alter tactile sensation, making a gentle touch perceived as painful. The molecular mechanisms mediating this alteration in sensation, called mechanical allodynia, are not completely understood. Murthy et al. and Szczot et al. discovered that PIEZO2 ion channels expressed in sensory neurons are required for the development of mechanical allodynia in mice and humans. The authors independently reached this conclusion using different animal models. Szczot et al. extended the discovery to humans, showing that PIEZO2 loss of function resulted in failure to develop mechanical allodynia. These two studies suggest that local inhibition of PIEZO2 ion channels might be effective for treating mechanical allodynia.
Abstract
The brush of a feather and a pinprick are perceived as distinct sensations because they are detected by discrete cutaneous sensory neurons. Inflammation or nerve injury can disrupt this sensory coding and result in maladaptive pain states, including mechanical allodynia, the development of pain in response to innocuous touch. However, the molecular mechanisms underlying the alteration of mechanical sensitization are poorly understood. In mice and humans, loss of mechanically activated PIEZO2 channels results in the inability to sense discriminative touch. However, the role of Piezo2 in acute and sensitized mechanical pain is not well defined. Here, we showed that optogenetic activation of Piezo2-expressing sensory neurons induced nociception in mice. Mice lacking Piezo2 in caudal sensory neurons had impaired nocifensive responses to mechanical stimuli. Consistently, ex vivo recordings in skin-nerve preparations from these mice showed diminished Aδ-nociceptor and C-fiber firing in response to mechanical stimulation. Punctate and dynamic allodynia in response to capsaicin-induced inflammation and spared nerve injury was absent in Piezo2-deficient mice. These results indicate that Piezo2 mediates inflammation- and nerve injury–induced sensitized mechanical pain, and suggest that targeting PIEZO2 might be an effective strategy for treating mechanical allodynia.