2025-05-01 東京科学大学
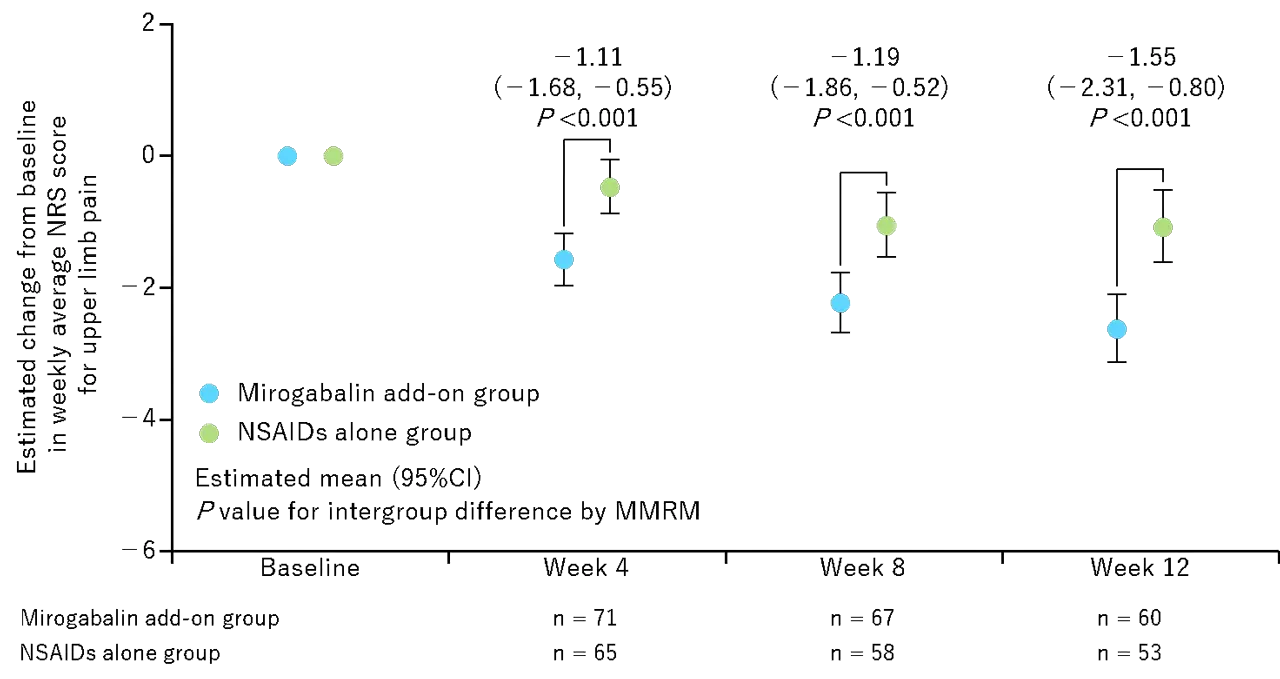
図1. 上肢痛NRSの投与開始時から12週にわたる推定変化
<関連情報>
- https://www.isct.ac.jp/ja/news/itumhrey7e4h
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1408&prevId=&key=e03ff3eeff998fd62014152a04b220c2.pdf
- https://link.springer.com/article/10.1007/s40122-025-00722-w
頚椎症性神経根症による神経障害性疼痛患者におけるミロガバリンの有効性と安全性: Miro-Cens、無作為化比較介入試験 Efficacy and Safety of Mirogabalin in Patients with Neuropathic Pain Due to Cervical Spondylotic Radiculopathy: Miro-Cens, A Randomized, Controlled, Interventional Study
Takashi Hirai,Atsushi Okawa,Hiroshi Takahashi,Kazuhito Shiosakai & Toshitaka Yoshii On behalf of the Miro-Cens investigators
Pain and Therapy Published:12 April 2025
DOI:https://doi.org/10.1007/s40122-025-00722-w
Abstract
Introduction
There are few studies of pharmacotherapy of neuropathic pain in cervical spondylotic radiculopathy (CSR). Miro-Cens aimed to examine the efficacy and safety of mirogabalin for treating pain in patients with CSR on non-steroidal anti-inflammatory drugs (NSAIDs), compared with NSAIDs alone.
Methods
Miro-Cens was a 12-week, multicenter, randomized, controlled, open-label, interventional study in Japan. Eligible patients with CSR having upper limb pain (visual analog scale score ≥ 40 mm) were randomly assigned in a 1:1 ratio to the mirogabalin add-on to NSAIDs group and the NSAIDs alone group. The primary endpoint was the change in the weekly average numerical rating scale (NRS) score for upper limb pain from baseline at Week 12.
Results
The mirogabalin add-on group and NSAIDs alone group included 72 and 70 patients, respectively. The mirogabalin add-on group had a significantly greater reduction in the NRS score for upper limb pain than the NSAIDs alone group: estimated changes from baseline at Week 12, – 2.63 [95% confidence interval (CI) – 3.14, – 2.11] in the mirogabalin add-on group; – 1.07 (- 1.62, – 0.53) in the NSAIDs alone group; intergroup difference, – 1.55 (- 2.31, – 0.80; p < 0.001). The responder rate on the NRS score at Week 12 was significantly higher in the mirogabalin add-on group than in the NSAIDs alone group: ≥ 30% improvement, 71.7% vs. 39.6%; ≥ 50% improvement, 58.3% vs. 22.6% (both p < 0.001). The frequent treatment-emergent adverse drug reactions in the mirogabalin add-on group were the known ones (somnolence and dizziness), with most being mild or moderate in severity.
Conclusion
In patients with CSR, combination therapy with mirogabalin and NSAIDs significantly improved neuropathic pain compared with NSAID monotherapy. No new safety concerns were identified, although caution should be exercised regarding somnolence and dizziness. These findings suggest that concomitant use of mirogabalin with NSAIDs could be tolerable and a novel treatment option for CSR patients with insufficient analgesic effects on NSAIDs.
Trial Registration Number
jRCTs031210629.