2025-05-07 清華大学
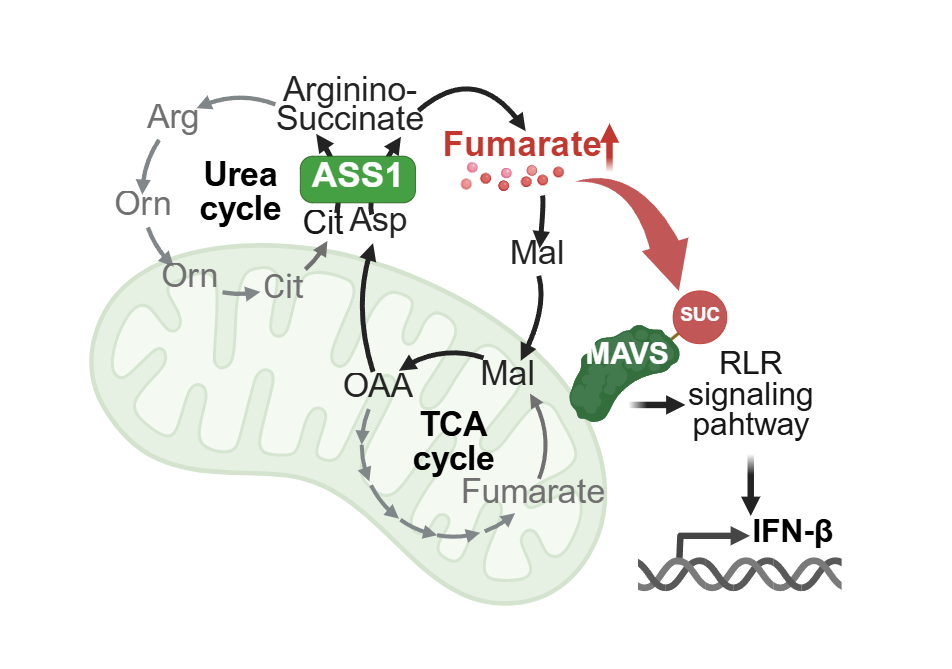 Figure 1. Viral infection-induced metabolic reprogramming promotes fumarate production to enhance antiviral innate immune responses.
Figure 1. Viral infection-induced metabolic reprogramming promotes fumarate production to enhance antiviral innate immune responses.
<関連情報>
- https://www.tsinghua.edu.cn/en/info/1245/14232.htm
- https://www.nature.com/articles/s41564-025-01985-x
代謝的リモデリングは、抗ウイルス防御としてマクロファージにおいてアスパラギン酸-アルギニノコハク酸シャントを介してフマル酸を産生する Metabolic remodelling produces fumarate via the aspartate–argininosuccinate shunt in macrophages as an antiviral defence
Wenjun Xia,Youxiang Mao,Ziyan Xia,Jie Cheng & Peng Jiang
Nature Microbiology Published:18 April 2025
DOI:https://doi.org/10.1038/s41564-025-01985-x
Abstract
Metabolic remodelling underpins macrophage effector functions in response to various stimuli, but the mechanisms involved are unclear. Here we report that viral-infection-induced inflammatory stimulation causes a rewiring of the urea cycle and the tricarboxylic acid cycle metabolism in macrophages to form a cyclic pathway called the aspartate–argininosuccinate (AAS) shunt. Using RNA sequencing, unbiased metabolomics and stable isotope tracing, we found that fumarate generated from the AAS shunt is driven by argininosuccinate synthase (ASS1) in the cytosol and potentiates inflammatory effects. Genetic ablation of ASS1 reduces intracellular fumarate levels and interferon-β production, and mitochondrial respiration is also suppressed. Notably, viral challenge or fumarate esters enhance interferon-β production via direct succination of the mitochondrial antiviral signalling protein and activation of the retinoic acid-inducible gene-I-like receptor signalling. In addition to the vesicular stomatitis virus, the Sendai virus and influenza A virus can also exert these effects. In addition, patients with Ebola virus disease have increased ASS1 expression and ASS1-deficient mice show suppressed macrophage interferon responses to vesicular stomatitis virus infection. These findings reveal that fumarate can be produced from the viral inflammation-induced AAS shunt and is essential for antiviral innate immunity.