2025-05-07 カリフォルニア大学サンディエゴ校 (UCSD)
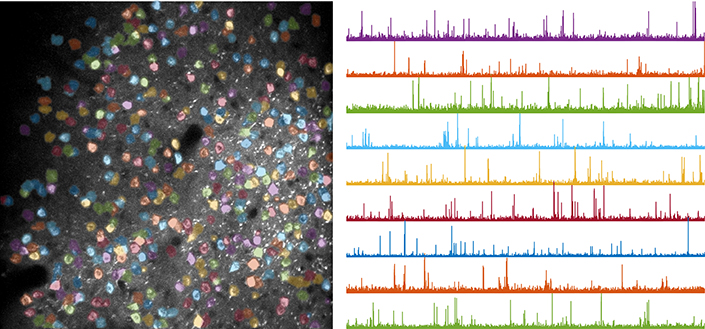
Neuronal activity traces reveal how brain circuits evolve as mice learn a motor task. Left: example field of view recorded during behavior; each color marks a different neuron. Right: activity traces from selected neurons.
<関連情報>
- https://today.ucsd.edu/story/neuroscientists-pinpoint-where-and-how-brain-circuits-are-reshaped-as-we-learn-new-movements
- https://www.nature.com/articles/s41586-025-08962-8
- https://www.science.org/doi/abs/10.1126/science.ads4706
運動学習は運動皮質への視床の影響を洗練する Motor learning refines thalamic influence on motor cortex
Assaf Ramot,Felix H. Taschbach,Yun C. Yang,Yuxin Hu,Qiyu Chen,Bobbie C. Morales,Xinyi C. Wang,An Wu,Kay M. Tye,Marcus K. Benna &Takaki Komiyama
Nature Published:07 May 2025
DOI:https://doi.org/10.1038/s41586-025-08962-8
Abstract
The primary motor cortex (M1) is central for the learning and execution of dexterous motor skills1,2,3, and its superficial layer (layers 2 and 3; hereafter, L2/3) is a key locus of learning-related plasticity1,4,5,6. It remains unknown how motor learning shapes the way in which upstream regions activate M1 circuits to execute learned movements. Here, using longitudinal axonal imaging of the main inputs to M1 L2/3 in mice, we show that the motor thalamus is the key input source that encodes learned movements in experts (animals trained for two weeks). We then use optogenetics to identify the subset of M1 L2/3 neurons that are strongly driven by thalamic inputs before and after learning. We find that the thalamic influence on M1 changes with learning, such that the motor thalamus preferentially activates the M1 neurons that encode learned movements in experts. Inactivation of the thalamic inputs to M1 in experts impairs learned movements. Our study shows that motor learning reshapes the thalamic influence on M1 to enable the reliable execution of learned movements.
学習中の樹状突起コンパートメント間で、生体内で異なるシナプス可塑性ルールが働いていることがわかった Distinct synaptic plasticity rules operate across dendritic compartments in vivo during learning
William J. Wright, Nathan G. Hedrick, and Takaki Komiyama
Science Published:17 Apr 2025
DOI:https://doi.org/10.1126/science.ads4706
Editor’s summary
The brain learns from experience through changes in synaptic weights. But how are specific synapses selected to undergo different forms of plasticity during learning? Wright et al. examined synaptic plasticity rules in different dendritic compartments of layer 2/3 pyramidal neurons of the mouse primary motor cortex (see the Perspective by Groisman and Letzkus). Strengthening of apical synapses depended on their correlated activity with their neighboring synapses and was independent of postsynaptic action potentials. By contrast, basal synapse strengthening was driven by activity coincidence with the postsynaptic action potentials, consistent with Hebbian mechanisms of plasticity. These different plasticity rules suggest functional specializations within individual neurons. Apical plasticity drives the formation of functional clusters of learning-related synapses for nonlinear integration, whereas basal plasticity favors the formation of Hebbian ensembles for reliable pattern completion. —Peter Stern
Abstract
Synaptic plasticity underlies learning by modifying specific synaptic inputs to reshape neural activity and behavior. However, the rules governing which synapses will undergo different forms of plasticity in vivo during learning and whether these rules are uniform within individual neurons remain unclear. Using in vivo longitudinal imaging with single-synapse resolution in the mouse motor cortex during motor learning, we found that apical and basal dendrites of layer 2/3 (L2/3) pyramidal neurons showed distinct activity-dependent synaptic plasticity rules. The strengthening of apical and of basal synapses is predicted by local coactivity with nearby synapses and activity coincident with postsynaptic action potentials, respectively. Blocking postsynaptic spiking diminished basal synaptic potentiation without affecting apical plasticity. Thus, individual neurons use multiple activity-dependent plasticity rules in a compartment-specific manner in vivo during learning.