2025-04-16 メモリアル・スローン・ケタリングがんセンター (MSKCC)
<関連情報>
- https://www.mskcc.org/news/potential-treatment-for-parkinsons-using-investigational-cell-therapy-shows-early-promise
- https://www.nature.com/articles/s41586-025-08845-y
パーキンソン病に対するhES細胞由来ドーパミン作動性ニューロンの第I相試験 Phase I trial of hES cell-derived dopaminergic neurons for Parkinson’s disease
V. Tabar,H. Sarva,A. M. Lozano,A. Fasano,S. K. Kalia,K. K. H. Yu,C. Brennan,Y. Ma,S. Peng,D. Eidelberg,M. Tomishima,S. Irion,W. Stemple,N. Abid,A. Lampron,L. Studer & C. Henchcliffe
Nature Published:16 April 2025
DOI:https://doi.org/10.1038/s41586-025-08845-y
Abstract
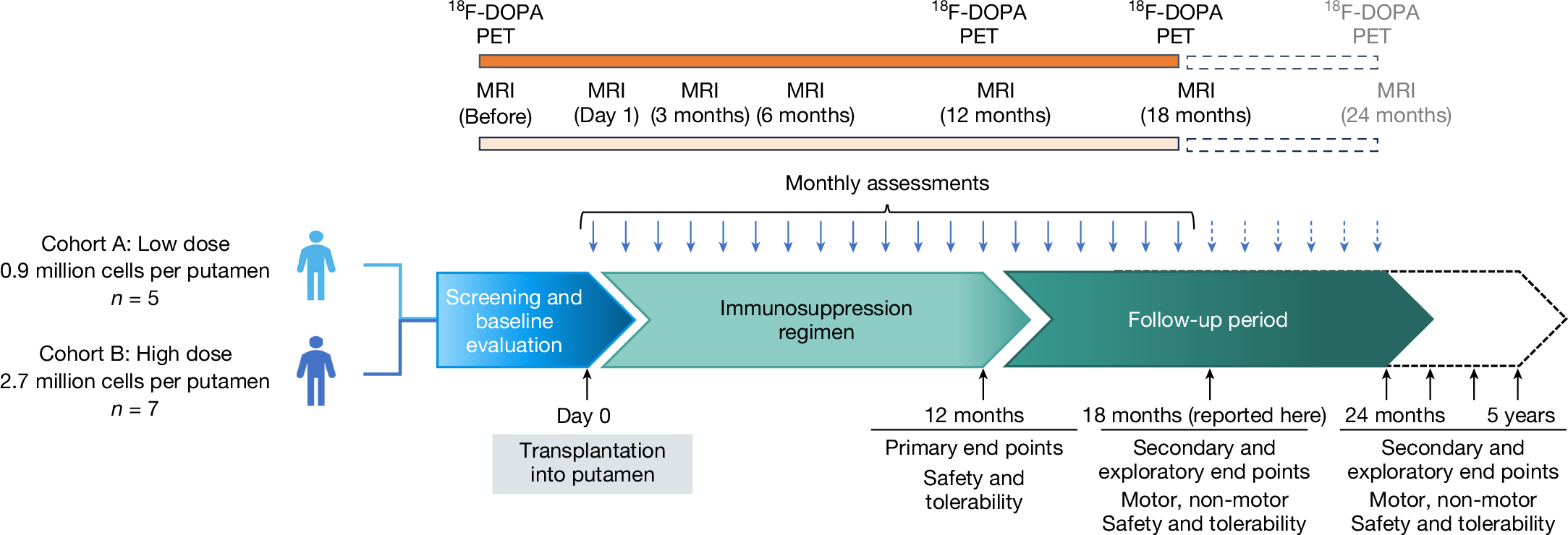
Parkinson’s disease is a progressive neurodegenerative condition with a considerable health and economic burden1. It is characterized by the loss of midbrain dopaminergic neurons and a diminished response to symptomatic medical or surgical therapy as the disease progresses2. Cell therapy aims to replenish lost dopaminergic neurons and their striatal projections by intrastriatal grafting. Here, we report the results of an open-label phase I clinical trial (NCT04802733) of an investigational cryopreserved, off-the-shelf dopaminergic neuron progenitor cell product (bemdaneprocel) derived from human embryonic stem (hES) cells and grafted bilaterally into the putamen of patients with Parkinson’s disease. Twelve patients were enrolled sequentially in two cohorts—a low-dose (0.9 million cells, n = 5) and a high-dose (2.7 million cells, n = 7) cohort—and all of the participants received one year of immunosuppression. The trial achieved its primary objectives of safety and tolerability one year after transplantation, with no adverse events related to the cell product. At 18 months after grafting, putaminal 18Fluoro-DOPA positron emission tomography uptake increased, indicating graft survival. Secondary and exploratory clinical outcomes showed improvement or stability, including improvement in the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III OFF scores by an average of 23 points in the high-dose cohort. There were no graft-induced dyskinesias. These data demonstrate safety and support future definitive clinical studies.