2025-05-08 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/nuclear-medicine-better-diagnosis-and-therapy-for-brain-metastases.html
- https://www.nature.com/articles/s41591-025-03633-7
アミノ酸PETイメージングに基づく脳転移の反応評価のためのRANO基準 RANO criteria for response assessment of brain metastases based on amino acid PET imaging
Nathalie L. Albert,Norbert Galldiks,Benjamin M. Ellingson,Martin J. van den Bent,Susan M. Chang,Francesco Cicone,Eng-Siew Koh,Ian Law,Emilie Le Rhun,Maximilian J. Mair,Jan-Michael Werner,Anna S. Berghoff,Julia Furtner,Giuseppe Minniti,Andrew M. Scott,Susan C. Short,Jana Ivanidze,Derek R. Johnson,Bogdana Suchorska,Nelleke Tolboom,Joerg-Christian Tonn,Antoine Verger,Eva Galanis,Priscilla K. Brastianos,… Matthias Preusser
Nature Medicine Published:08 May 2025
DOI:https://doi.org/10.1038/s41591-025-03633-7
Abstract
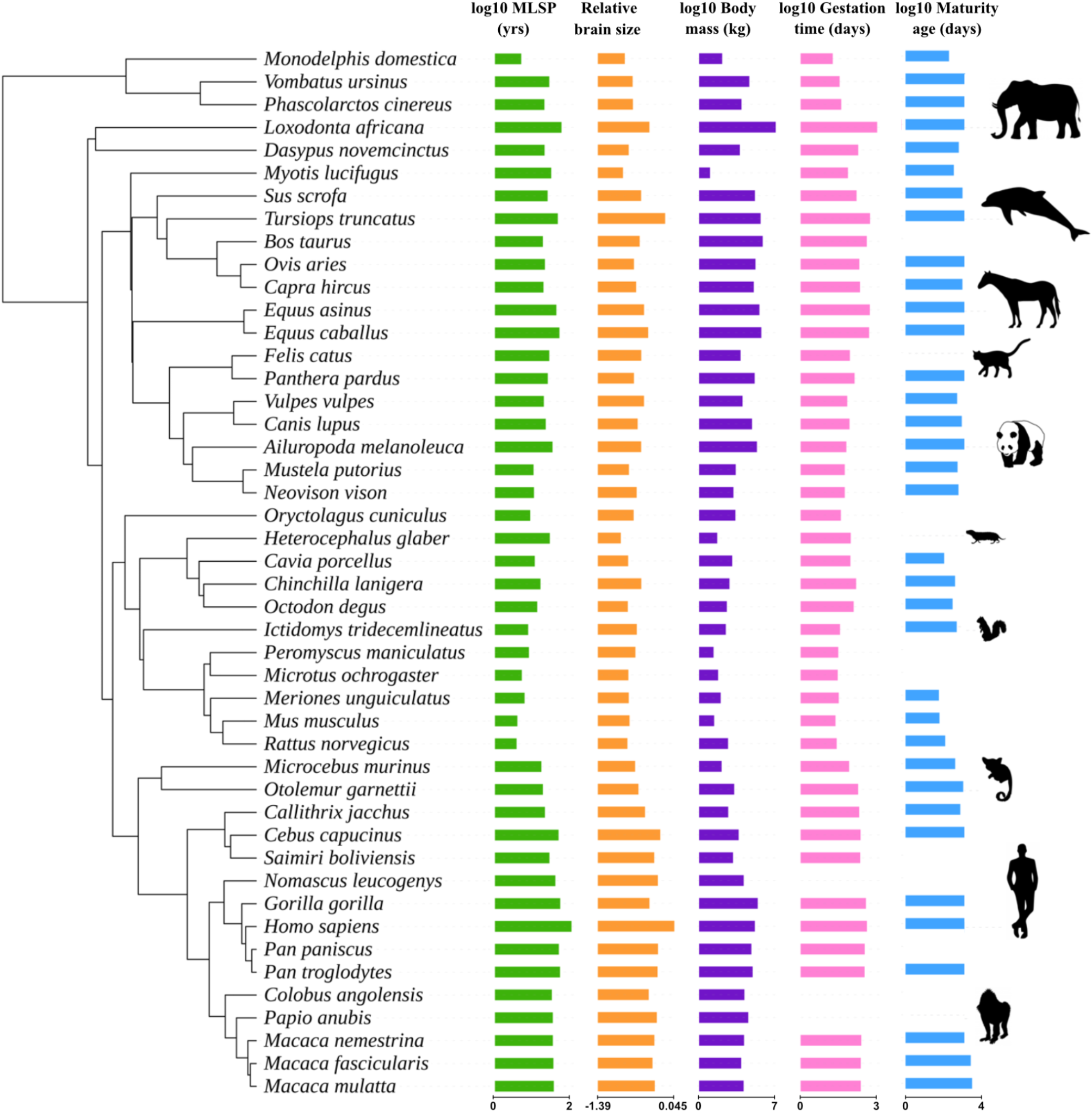
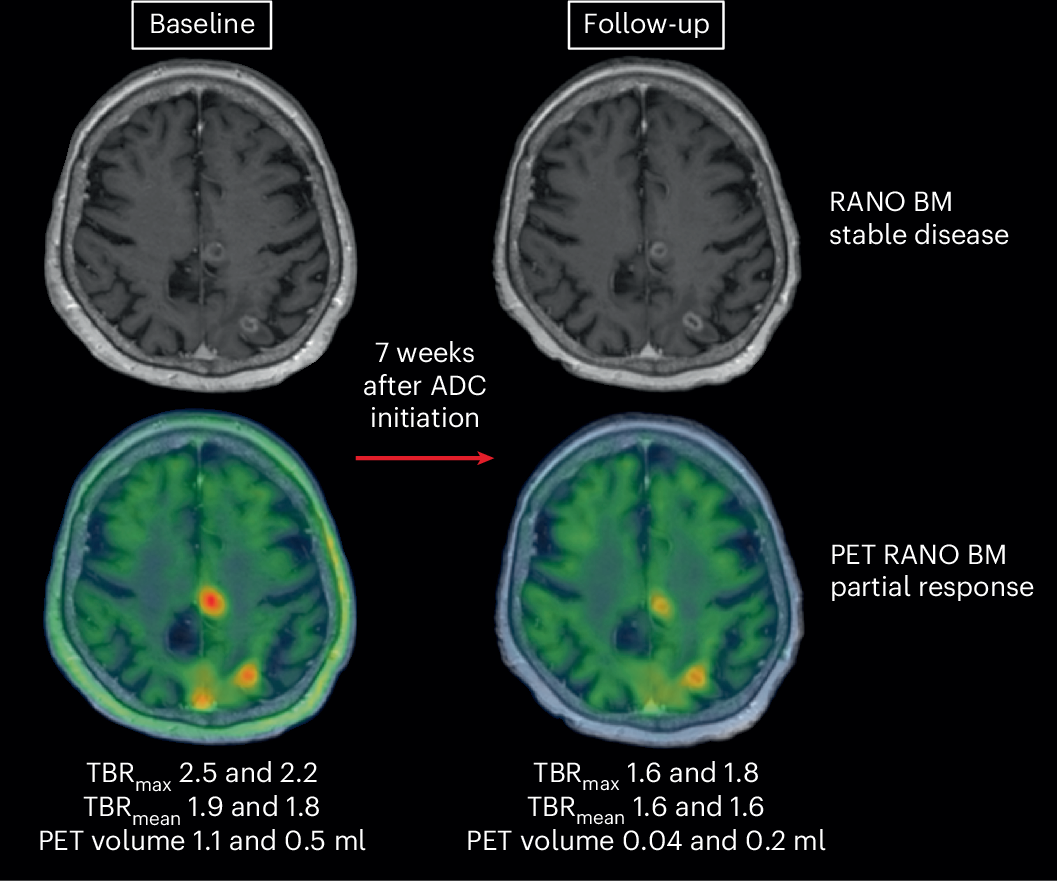
Novel diagnostic and therapeutic opportunities are needed to improve medical care and outcome of patients with brain metastases, a frequent and severe complication of several cancer types. Currently, magnetic resonance imaging (MRI) is the primary method used for detection, treatment planning and disease monitoring in patients with brain metastases, but this method has limitations. These limitations mean that MRI can inform on lesion size but cannot directly measure the activity or viability of tumor tissue. Positron emission tomography (PET) imaging, however, can visualize metabolically active tumor cells and is therefore increasingly incorporated into cancer care to assess tumor burden and response to treatment. Here, we define the PET Response Assessment in Neuro-Oncology (RANO) for brain metastasis (BM) 1.0 criteria for metabolic response assessment of brain metastases using amino acid PET. By introducing an innovative endpoint for next-generation clinical trials, the PET RANO BM 1.0 criteria aim to facilitate development of novel therapies for patients with brain metastases.