2025-05-20 東京科学大学
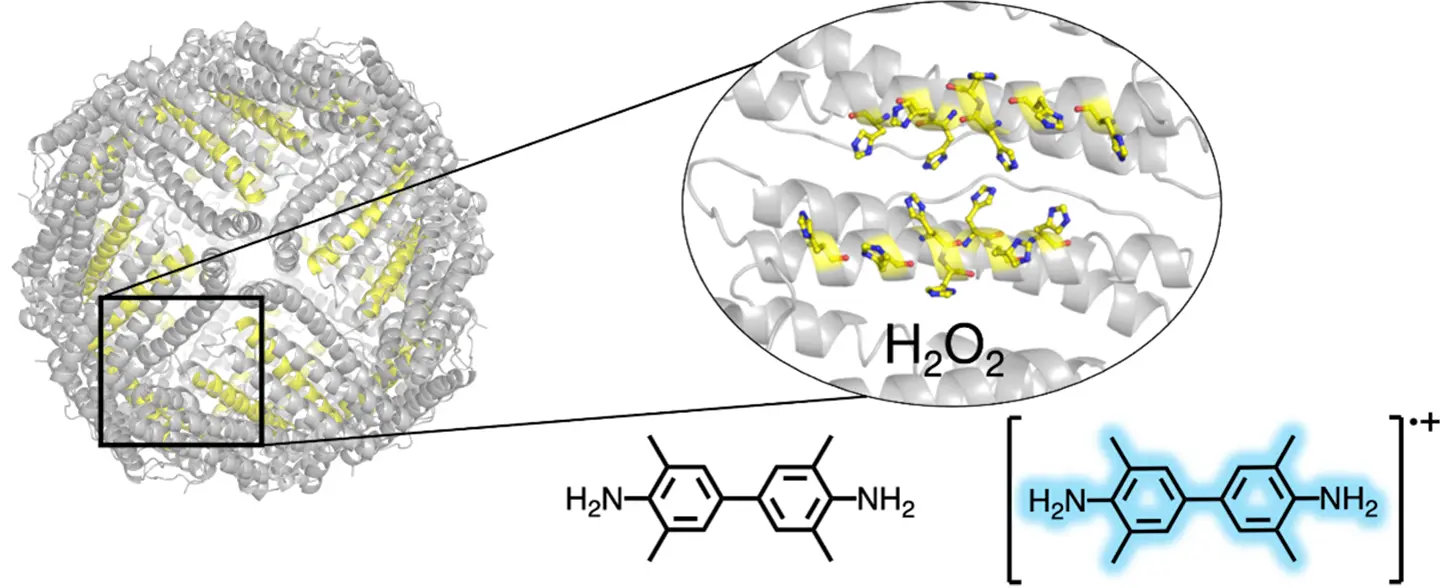 図1. タンパク質カゴであるフェリチンの中で生じる、過酸化水素が関与するペルオキシダーゼ様反応。
図1. タンパク質カゴであるフェリチンの中で生じる、過酸化水素が関与するペルオキシダーゼ様反応。
<関連情報>
- https://www.isct.ac.jp/ja/news/oqyl38imx2mx
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202504608
フェリチンケージを用いて設計した金属を含まない人工ペルオキシダーゼによるバイオインスパイアード触媒反応 An Artificial Metal-Free Peroxidase Designed Using a Ferritin Cage for Bioinspired Catalysis
Jiaxin Tian, Basudev Maity, Tadaomi Furuta, Tiezheng Pan, Takafumi Ueno
Angewandte Chemie International Edition Published: 24 April 2025
DOI:https://doi.org/10.1002/anie.202504608
Abstract
Developing artificial enzymes is challenging because it requires precise design of active sites with well-arranged amino acid residues. Histidine-rich oligopeptides have been recently shown to exhibit peroxidase-mimetic activities, but their catalytic function relies on maintaining unique supramolecular structures. This work demonstrates the design of a specific array of histidine residues on the internal surface of the ferritin cage to function as an active center for catalysis. The crystal structures of the ferritin mutants revealed histidine–histidine interactions, forming well-defined histidine clusters (His-clusters). These mutants exhibit peroxidase-mimetic activities by oxidizing 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of hydrogen peroxide. Molecular dynamics simulations further highlight the co-localization of TMB and hydrogen peroxide at the histidine-rich clusters, indicating that the confined environment of the ferritin cage enhances their interactions. This study presents a simple yet effective approach to design metal-free artificial enzymes, paving the way for innovations in bioinspired catalysis.