2025-05-26 北海道大学,熊本大学,株式会社RAINBOW
<関連情報>
- https://www.hokudai.ac.jp/news/2025/05/post-1892.html
- https://www.hokudai.ac.jp/news/pdf/250526_pr3.pdf
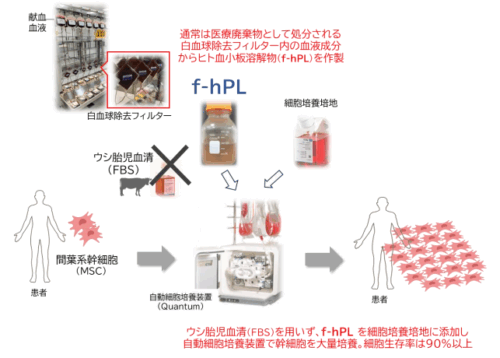
白血球除去フィルター由来の血小板融解物は幹細胞増殖に有益な材料となる Human platelet lysate produced from leukoreduction filter contents enables sufficient MSC growth
Shinobu Wakamoto,Tomoko Furukawa,Masahito Kawabori,Mitsuaki Akino,Shiho Kato,Hisae Fuse,Sumio Ohtsuki,Yoshihiro Torimoto,Miki Fujimura &Shuichi Kino
Stem Cell Research & TherapyPublished:23 April 2025
DOI:https://doi.org/10.1186/s13287-025-04329-y
Abstract
Background
Stem cell therapy holds significant potential for promoting recovery, with numerous products currently under development. Blood-derived supplements are often essential for successful stem cell expansion, with fetal bovine serum (FBS) being the most commonly used supplement. However, FBS has drawbacks, including the risk of immune responses, ethical concerns about animal welfare, and potential zoonotic infections. Human platelet lysate (hPL), derived from lysed platelets, contains various growth factors and has been proposed as an alternative to FBS. However, obtaining sufficient human platelets for clinical use remains challenging. Leukoreduction filters, used during blood transfusion manufacturing to remove leukocytes, also retain significant amounts of platelets and plasma. This study investigates the feasibility and efficacy of filter-derived hPL (f-hPL) for mesenchymal stem cell (MSC) expansion.
Methods
Leukoreduction filters were collected after their use in the manufacturing of whole blood transfusion products. Each filter was reverse-perfused with saline to extract residual blood contents. Platelets (f-platelet) and supernatant were separated by multiple centrifugation steps. f-Platelet were lysed with varying concentrations of fresh frozen plasma (FFP) to determine the optimal protein concentration for the lysate solution. Then, plasma left in the leukoreduction filters were used to generate lysate solution (f-plasma) at optimal protein concentration. f-Platelet (1.1 × 109/mL) and f-plasma (27 mg/mL protein) were combined in a freezing bag and subjected to three freeze-thaw cycles to produce f-hPL. Both small- and large-scale f-hPL were manufactured, and MSCs expansion and quality assessments were perfomed to evaluate the efficacy of f-hPL.
Results
A total of 3.5 ± 0.6 × 1010 f-platelets were obtained from a single leukoreduction filter, yielding a collection efficiency of 37.1 ± 5.3%. The optimal protein concentration of lysate solution for cell expansion was > 27 mg/mL. Subsequently, six leukoreduction filters used to produce enough f-platelet and p-plasma for 100 mL of f-hPL. MSCs cultured in medium supplemented with 10% f-hPL demonstrated superior expansion, with cell proliferation rates 20% higher than those observed with commercial hPL and 300% higher than those cultured with FBS. The expanded MSCs met the International Society for Cell & Gene Therapy criteria for cell surface markers and differentiation potential. MSCs expanded with f-hPL expressed similar to or higher amounts of hepatocyte growth factor compared with those cultured with FBS and human AB serum. Furthermore, f-hPL significantly enhanced cell proliferation up to P12 and effectively prevented cell senescence.
Conclusion
f-hPL derived from leukoreduction filters demonstrated strong capacity for MSCs expansion. The use of discarded blood materials for regenerative medicine represents a sustainable and efficient approach, with significant therapeutic potential.

