2025-06-10 国立国際医療研究所,北海道大学,金沢大学
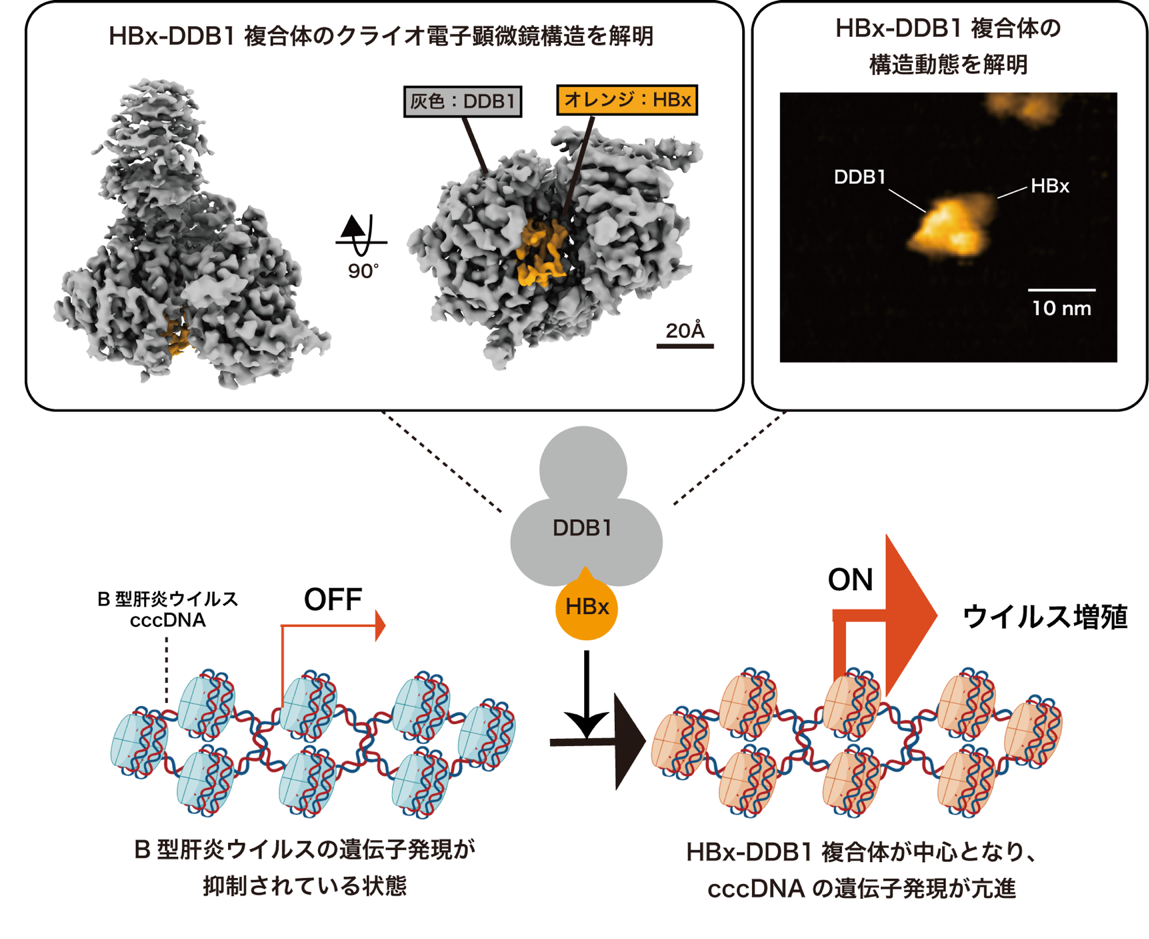
国立健康危機管理研究機構、北海道大学、金沢大学の研究チームは、B型肝炎ウイルス(HBV)の持続感染を担うウイルスタンパク質HBxとヒト因子DDB1の複合体構造をクライオ電子顕微鏡で初めて解明しました。さらに、高速原子間力顕微鏡により、HBxの球状かつ柔軟な構造が多様な宿主因子との相互作用を可能にする動的性質を持つことを可視化。HBxは転写抑制因子SMC5/6複合体の構成因子と結合・分解し、ウイルス遺伝子転写を活性化します。この知見は、HBVの遺伝子発現機構と治療標的探索に貢献し、慢性肝炎治療の新戦略に道を開くものです。
<関連情報>
- https://www.ri.jihs.go.jp/topics/release/2025/20250613.html
- https://www.ri.jihs.go.jp/topics/release/2025/pdf/202506_01.pdf
- https://www.pnas.org/doi/10.1073/pnas.2421325122
B型肝炎ウイルスxタンパク質とDDB1との複合体の構造基盤 Structural basis of the hepatitis B virus x protein in complex with DDB1
Hiroki Tanaka, Joao Diogo Dias, Basile Jay, +11 , and Shinichi Machida
Proceedings of the National Academy of Sciences Published:June 13, 2025
DOI:https://doi.org/10.1073/pnas.2421325122
Significance
HBx plays a pivotal role in HBV replication by enhancing cccDNA transcription. However, the HBx structure remains unsolved, representing a significant gap in knowledge. Here, we show the cryo-EM structure of HBx in complex with DDB1, which is a key complex for cccDNA transcription. In addition, HS-AFM revealed the dynamics of HBx in complex with DDB1. Our findings provide a comprehensive view of the structural, dynamic, and functional aspects of HBx and elucidate the key role of HBx in viral replication.
Abstract
A cure for chronic hepatitis B requires eliminating or permanently silencing covalently closed circular DNA (cccDNA). A pivotal target of this approach is the hepatitis B virus (HBV) X protein (HBx), which is a key factor that promotes transcription from cccDNA. However, the HBx structure remains unsolved. Here, we present the cryoelectron microscopy structure of HBx in complex with DDB1, which is an essential complex for cccDNA transcription. In this structure, hydrophobic interactions within HBx were identified, and mutational analysis highlighted their importance in the HBV life cycle. Our biochemical analysis revealed that the HBx–DDB1 complex directly interacts simultaneously with NSE3, which is a component of the SMC5/6 complex, and Spindlin1. Additionally, HBx–DDB1 complex dynamics were explored via high-speed atomic force microscopy. These findings provide comprehensive insights into the structure and function of HBx in HBV replication.