2025-06-10 中国科学院(CAS)
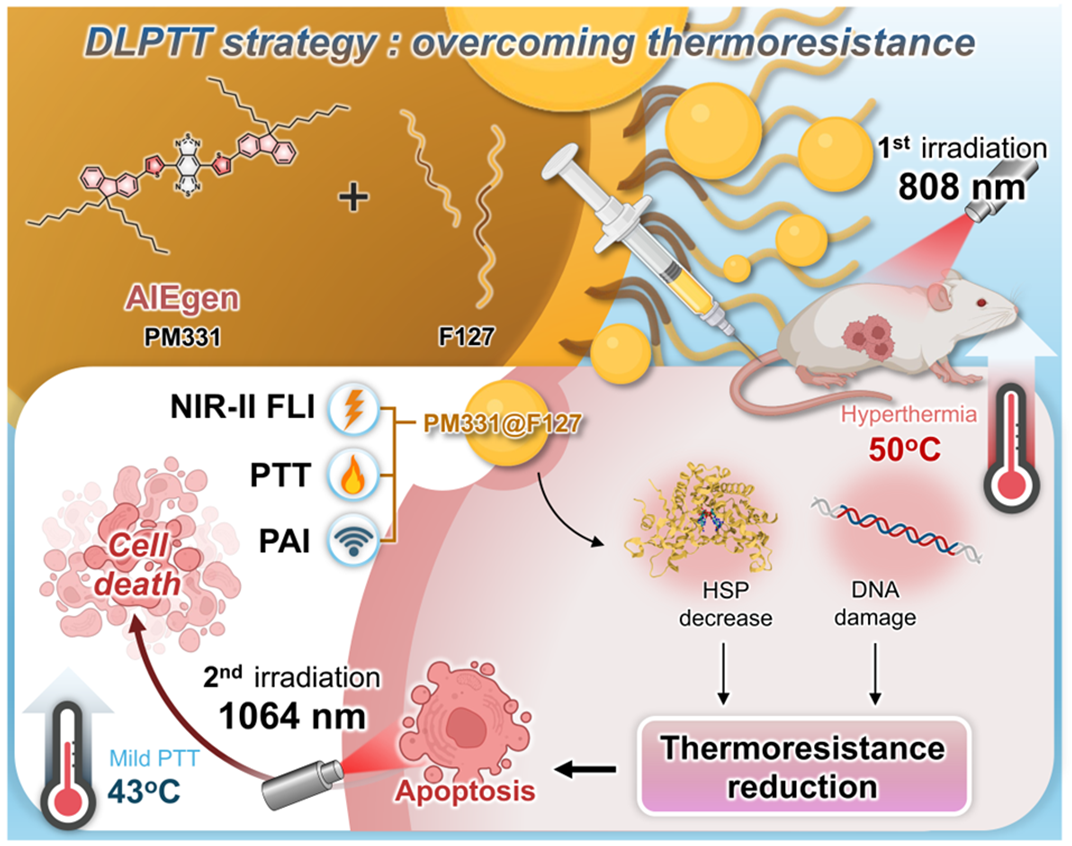
Schematic illustration of the DLPTT strategy designed to address the limitations of PTT. (Image by SIAT)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202506/t20250609_1045236.shtml
- https://www.pnas.org/doi/10.1073/pnas.2503574122
光熱療法のアキレス腱を回避する「808nmと1,064nm」のデュアルレーザー戦略 Dual-laser “808 and 1,064 nm” strategy that circumvents the Achilles’ heel of photothermal therapy
Qihang Ding, Jiqiang Liu, Yue Wang, +6 , and Jong Seung Kim
Proceedings of the National Academy of Sciences Published:June 9, 2025
DOI:https://doi.org/10.1073/pnas.2503574122
Significance
Photothermal therapy (PTT) has recently garnered attention within the scientific community as a possible cancer therapy. However, the risk of damaging healthy tissue due to overheating or failure to ablate effectively the tumors remains a challenge in the clinical development of PTT. The present study was designed to address this need. It relies on Pluronic F127-based NPs containing a small molecule PM331 with recognized aggregation-induced emission features. These NPs showed good thermal conversion efficiencies of 40% and 66% when excited at 808 nm and 1,064 nm, respectively. They also absorb well in the second near-infrared (NIR-II) window. Leveraging these characteristics, we developed a strategy called dual-laser PTT that integrates the benefits of traditional high-temperature and low-temperature PTT.
Abstract
Breast cancer has now overtaken lung cancer as the “world’s leading cancer,” yet detecting and implementing effective therapies remains a significant challenge. Substantial advances have been made in photothermal therapy (PTT), where photosensitizers use photonic energy to induce localized hyperthermia for cancer eradication. This pioneering approach is gaining traction in clinical settings. However, traditional PTT faces inherent limitations, including the risk of damage to neighboring healthy tissues and potential inflammatory responses due to overheating. Drawing inspiration from the distinct characteristics of aggregation-induced emission the small molecule, PM331, was chosen for study. This donor–acceptor–donor system displays good photothermal conversion efficiencies (40% and 66%) upon excitation at 808 nm and 1,064 nm, respectively. It is also characterized by attractive optical features in the second near-infrared (NIR-II) window. Using nanoparticles containing PM331, PM331@F127, we have developed a PTT strategy, termed dual-laser PTT (DLPTT), that involves successive excitation using 808 nm and 1,064 nm lasers guided by both NIR-II fluorescence and photoacoustic imaging. The DLPTT strategy involves two steps. First, it initiates DNA damage and downregulates heat shock protein expression as the result of an initial brief irradiation with an 808 nm laser. This is then followed by irradiation with a 1,064 nm laser to ablate tumor cells while minimizing inflammation and harm to surrounding healthy tissues. Based on the findings reported here, we suggest that DLPTT could represent an attractive approach to precision medicine and one that could make PTT more amenable to clinical implementation.

