2025-06-04 ウィスコンシン大学マディソン校(UW-Madison)
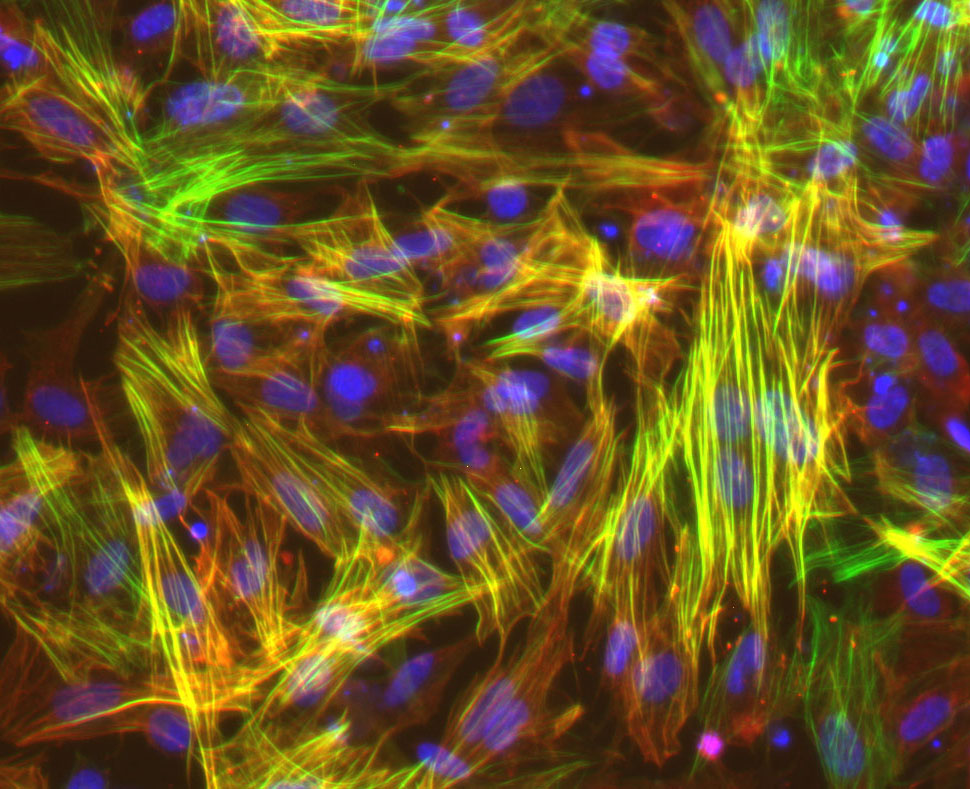
UW–Madison researchers studied smooth muscle cells like these, derived from induced pluripotent stem cells, to show that long-mysterious variations in an area of our genome harden blood vessels, puting some people at higher risk for cardiovascular disease. Image courtesy Lo Sardo Lab/UW–Madison
<関連情報>
- https://news.wisc.edu/uw-madison-researchers-find-hidden-genetic-clues-upping-cardiovascular-disease-risk/?srp
- https://www.ahajournals.org/doi/10.1161/ATVBAHA.124.322045
9p21.3冠動脈疾患リスク遺伝子座が血管平滑筋細胞を骨軟化状態へ誘導する The 9p21.3 Coronary Artery Disease Risk Locus Drives Vascular Smooth Muscle Cells to an Osteochondrogenic State
Elsa Salido, Carolina de Medeiros Vieira, Jose Verdezoto Mosquera, Rohan Zade, Parth Parikh, Shraddha Suryavanshi, Clint L. Miller, and Valentina Lo Sardo
Arteriosclerosis, Thrombosis, and Vascular Biology Published: 27 March 2025
DOI:https://doi.org/10.1161/ATVBAHA.124.322045
Abstract
BACKGROUND:
Genome-wide association studies have identified common genetic variants at ≈300 human genomic loci linked to coronary artery disease susceptibility. Among these genomic regions, the most impactful is the 9p21.3 coronary artery disease risk locus, which spans a 60-kb gene desert and encompasses ≈80 SNPs (single nucleotide polymorphism) in high linkage disequilibrium. Despite ≈2 decades since its discovery, the role of the 9p21.3 locus in cells of the vasculature remains incompletely resolved.
METHODS:
We differentiated induced pluripotent stem cells (iPSCs) from risk, nonrisk donors at 9p21.3, and isogenic knockouts into vascular smooth muscle cells (VSMCs). We performed single-cell transcriptomic profiling, including coembedding and comparison with publicly available human arterial data sets. We conducted functional characterization using migration and calcification assays and confirmed our findings on iPSC–VSMCs derived from additional donors. Finally, we used overexpression of ANRIL followed by gene expression analysis.
RESULTS:
We demonstrated that iPSC–VSMCs harboring the 9p21.3 risk haplotype preferentially adopt an osteochondrogenic state and show remarkable similarity to fibrochondrocytes from human artery tissue. The transcriptional profile and functional assessment of migration and calcification capacity across iPSC–VSMC lines from multiple donors concordantly resemble an osteochondrogenic state. Importantly, we identified numerous transcription factors driving different VSMC state trajectories. Additionally, we prioritized LIMCH1 and CRABP1 as signature genes critical for defining the risk transcriptional program. Finally, overexpression of a short isoform of ANRIL in 9p21.3 knockout cells was sufficient to induce the osteochondrogenic transcriptional signature.
CONCLUSIONS:
Our study provides new insights into the mechanism of the 9p21.3 risk locus and defines its previously undescribed role in driving a disease-prone transcriptional and functional state in VSMCs concordant with an osteochondrogenic-like state. Our data suggest that the 9p21.3 risk haplotype likely promotes arterial calcification, through altered expression of ANRIL, in a cell type–specific and cell-autonomous manner, providing insight into potential risk assessment and treatment for carriers.